Figure 4.
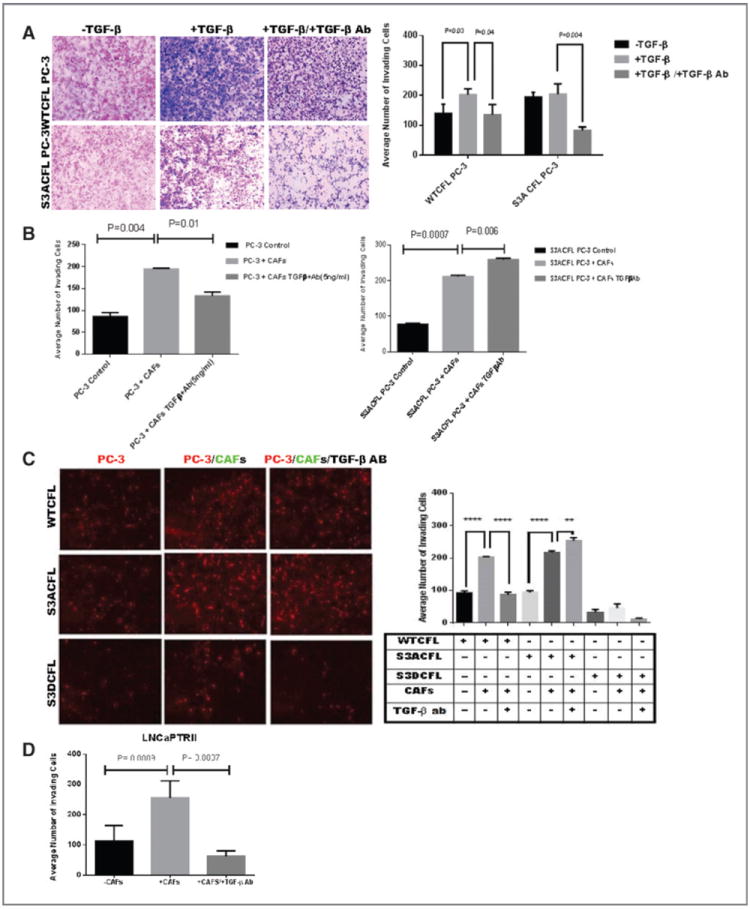
Cofilin navigates invasive response to TGF-β (from stroma microenvironment). A, the invasive response of prostate cancer cells to TGF-β assessed in the Matrigel assay. S3ACFL had no significant effect on PC-3 cell invasion (black barographs). In response to exogenous TGF-β, there was an increase in WTCFL PC-3 cell invasion potential, but not in S3ACFL cells (P = 0.03). In the presence of TGF-β–neutralizing antibody, there was a significant decrease in the invasion potential for both WTCFL and S3ACFL cells (P = 0.04 and 0.004, respectively). B, Matrigel invasion of WTCFL PC-3/CAFs (right) and S3ACFL PC-3/CAFs cocultures (left) after 24 hours. CAFs significantly increased prostate cancer cell invasion for both WTCFL and S3ACFL cells (P = 0.004 and 0.007. Continuous secretion of TGF-β by the reactive microenvironment (in the presence of TGF-β–neutralizing antibody) induced a further increase in the number of invading S3ACFL cells (P = 0.008), whereas it decreased WTCFL cell invasion. Values are the average from two independent experiments in triplicate. C, cocultures of WTCFL, S3ACFL, and S3DCFL with CAFs in the presence or absence of a neutralizing TGF-β antibody. Quantitative assessment of invading cells indicates that only active cofilin (S3A CFL) directs a further increase in TGF-β–mediated cell invasion (derived from CAFs). D, a significantly higher increase in cell invasion is stimulated by CAFs in cocultures with theLNCaP TβRII cells (highly responsive to TGF-β). This was abrogated by the presence of the neutralizing antibody against TGF-β. *, significant difference at P < 0.007.
