Figure 5.
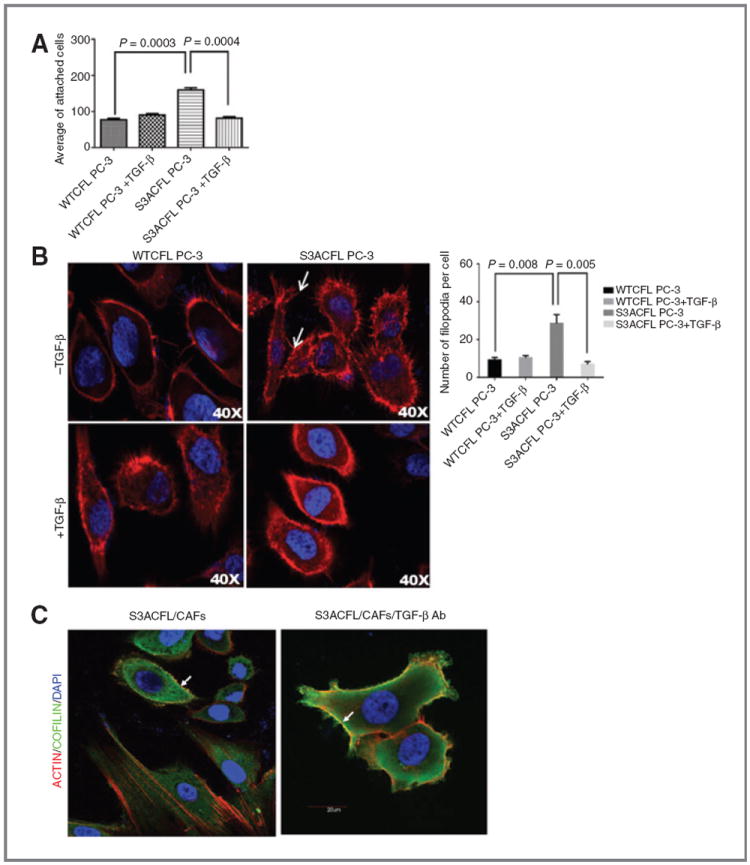
Active cofilin mediates prostate cancer cell adhesion via cytoskeletal remodeling, an effect impaired by TGF-β. A, effect of S3ACFL on prostate cancer cell adhesion. S3A mutation conferred a significant increase in cell adhesion to fibronectin compared with WTCFL cells (P = 0.0003). TGF-β significantly decreased in S3CFL cell adhesion (P = 0.0004), but not in WTCFL cells. Values shown are the mean (± SEM) of three independent experiments performed in triplicates. Statistical significance set at a P value of <0.005. B, active cofilin enhances filopodia formation; representative images of confocal microscopy (×40) show increased number of filopodia protrusions in S3ACFL PC-3 (arrows) compared with WTCFL cells. Treatment with TGF-β (5 ng/mL; 24 hours) decreased filopodia protrusions in S3ACFL cells. Filopodia quantitated in five random fields were examined for each cell line and values shown are mean ± SEM from three independent experiments (left). Statistical significance is defined at P < 0.01. C, S3ACFL active binding to F-actin at the leading edge of the cells is mediated by TGF-β. Cofilin colocalization with filopodia is dependent on TGF-β derived from surrounding stroma (CAFs). Images of cofilin/rhodamine phalloidin colocalization in S3ACFL prostate epithelial cancer cells cocultured with CAFs. Cofilin (green) colocalizes with filopodia protrusions (arrows). Loss of TGF-β (in the presence of neutralizing antibody) increases actin/cofilin colocalization (yellow) and filopodia protrusions in S3ACFL cells.
