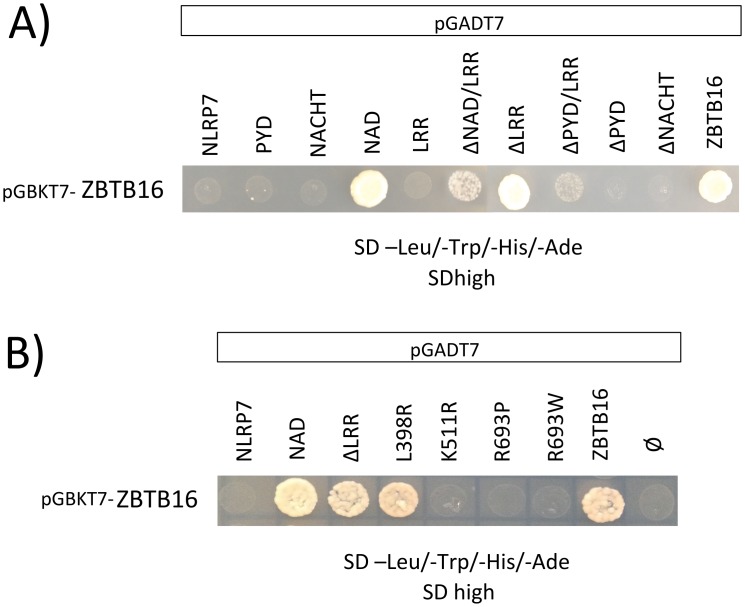
Fig 2. Yeast two-hybrid screen of full-length ZBTB16 against different NLRP7 deletion constructs and against full-length NLRP7 containing different HYDM1-causing mutations.
A. Yeast two-hybrid screen against the different NLRP7 constructs (fused to the GAL4 activation domain; pGADT7) using full-length ZBTB16 as bait (fused to the GAL4 binding domain; pGBKT7) performed with the Gal4-System (Clontech). ZBTB16 interacted with the highly reactive NAD domain and the LRR deleted constructs. An interaction of ZBTB16 with full-length NLRP7 failed. The self-interaction between full-length ZBTB16 served as positive control as it is already known that ZBTB16 forms a dimer by its N-terminal POZ/BTB domain [30]. B. Yeast two-hybrid mutation screen using ZBTB16 as bait (pGBKT7) against full-length NLRP7 containing one of the three HYDM1-causing mutations L398R (NACHT), R693P (LRR), R693W (LRR) or the NSV K511R (all pGADT7). The NACHT domain-associated mutation L398R resulted in the same strong interaction between full-length NLRP7 and full-length ZBTB16 as seen between NAD:ZBTB16 and ΔLRR:ZBTB16.