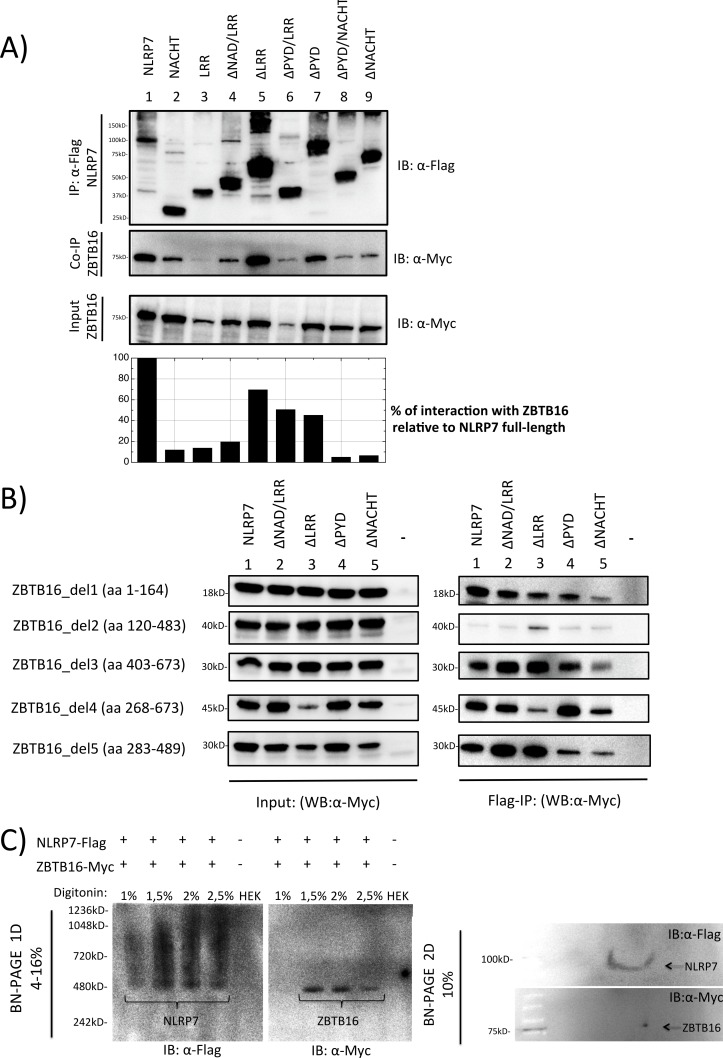
Fig 3. Co-Immunoprecipitation and Blue Native PAGE analysis between NLRP7 and ZBTB16.
A. Co-immunoprecipitation of transiently transfected ZBTB16 (Myc-tagged) and NLRP7 (Flag-tagged) in HEK293T cells. Immunoprecipitation was done using an anti-Flag specific antibody. Quantification of interaction between NLRP7 and ZBTB16 was determined relative to NLRP7 full-length. B. Co-immunoprecipitation of five ZBTB16 deletion constructs del1-5 (Myc-tagged) by immunoprecipitation of full-length NLRP7 (Lane 1) or one of four NLRP7 deletion constructs (lane 2–5; Flag-tagged) using an anti-Flag specific antibody. C. Blue Native gel electrophoresis of NLRP7 (Flag-tagged) and ZBTB16 (Myc-tagged) after transient transfection in HEK293T cells. Native protein extraction was performed using different concentrations of digitonin that is indicated above the picture. Upper panels: In the first dimension, the native Flag-NLRP7 appears as a smeary oligomer in a broad shift from 480–1000 kD (left side), while native ZBTB16-Myc was detected as a sharp band at 480 kD (right side). Lower panels: In the second dimension, the monomeric Flag-NLRP7 is visible as a thin line at 113kD, while ZBTB16-Myc occurs as individual spot at its expected size of 75 kD.