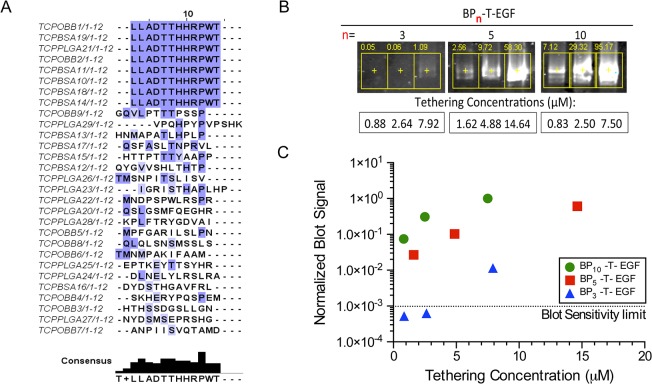
Fig 2. Identification of ßTCP-binding peptide and concatamerization of the sequence in EGF fusion proteins.
Sequence alignment of multiple panning experiments against ßTCP material using orthogonal blocking and panning against composite ßTCP-PLGA substrates showing that the consensus sequence in 8 of 29 clones is LLADTTHHRPWT (A). Anti-EGF immunoblot performed against scaffold-eluted BPn-T-EGF fusion protein with different binding peptide repeats (n = 3, 5 and 10) tethered at dilutions as indicated, illustrating greater tethering with increase n repeats of the binding peptide in the concatamer (B). Quantification of anti-EGF immunoblot signals depicted in 2B shows that the 10-mer repeat of the 12-amino acid ßTCP-binding peptide imparts the highest affinity binding to the BPn-T-EGF fusion protein (C).