Fig 1. Sav is ubiquitylated predominantly in the C-term half of the protein.
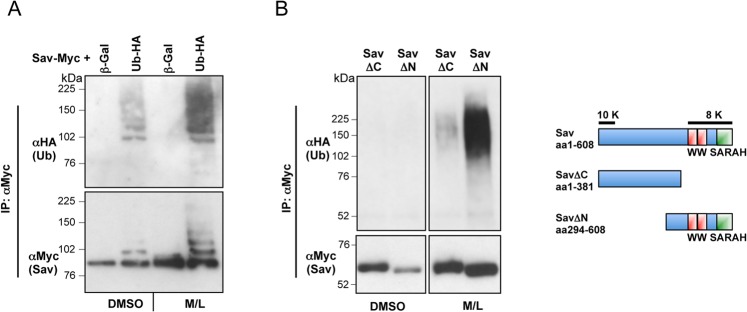
(A) Sav is stabilised and shows increased ubiquitylation in the presence of proteasome inhibitors (MG132 and LLnL). Myc-tagged Sav was expressed in S2 cells with either Ub-HA or ß-Galactosidase (ß-Gal) in the presence of proteasome inhibitors (MG132 and LLnL, M/L) or DMSO control. Sav ubiquitylation assay shows incorporation of HA-tagged ubiquitin. (B) Left panel: Sav ubiquitylation occurs in the C-terminal half of the protein. Sav deletions (∆C and ∆N) were tested for ubiquitylation as described above. Right panel: schematic representation of Sav protein structure and distribution of Lysine residues. Sav contains eighteen Lysine residues (K), which could serve as potential ubiquitylation sites: ten are located near the N-terminal end of the protein, whereas the remaining eight are located in the C-terminus.
