Fig 2. Gross observations of calvarial bone defect sites after 8 weeks treatment.
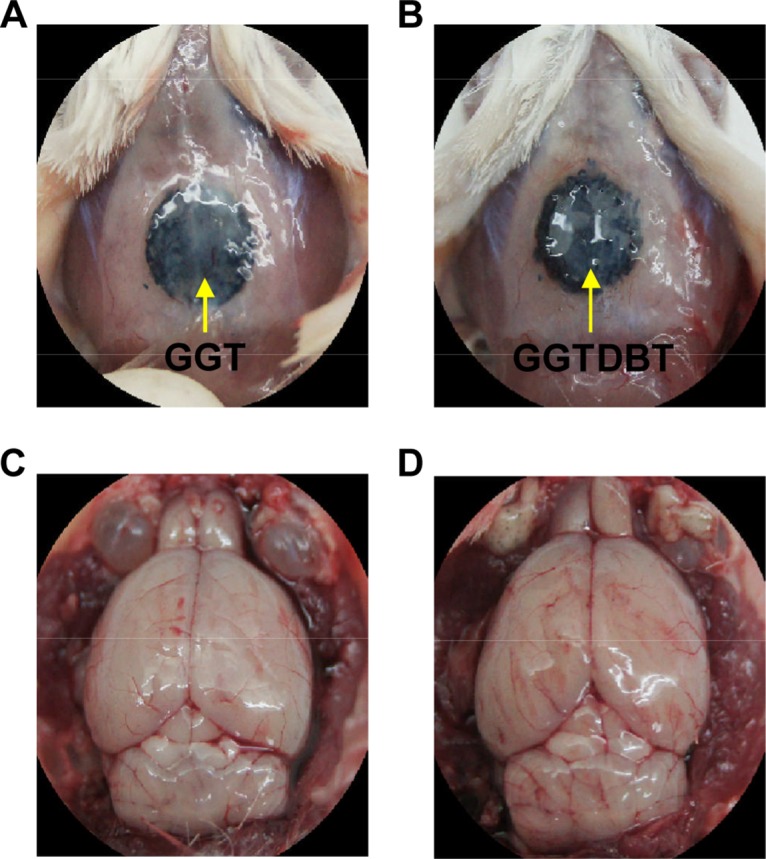
No evidence of infection, inflammation, hematoma, or necrosis was observed at the implant sites around the calvarial bone defects treated with (A) GGT and (B) GGTDBT composites. The brain tissues under the implantation sites exhibited no evidence of adverse tissue reaction to the (C) GGT and (D) GGTDBT composites.
