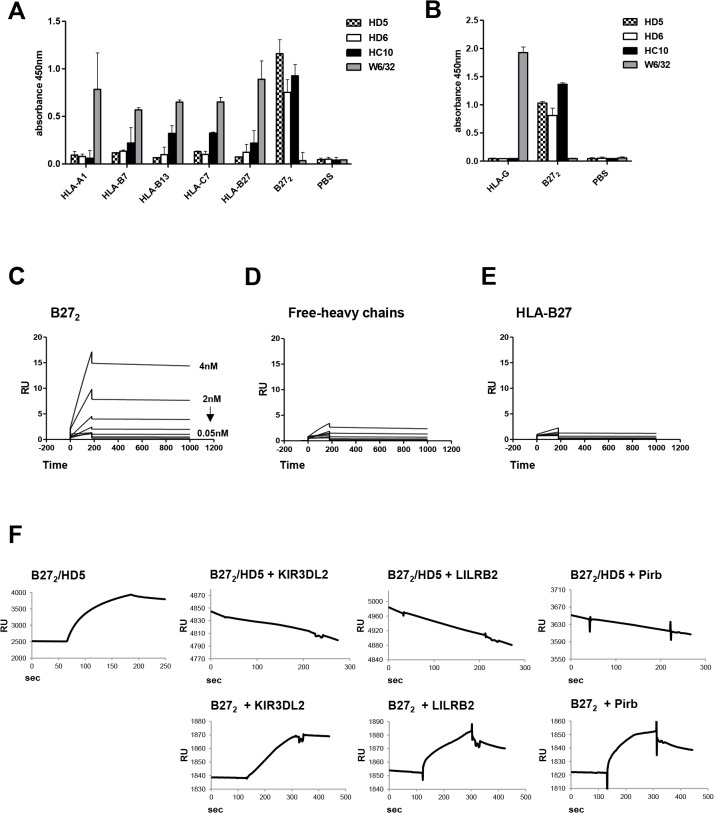
Fig 1. HD5 mAb is specific to B272, binds to recombinant B272, and blocks interaction with immunoregulatory cell receptors.
(A) ELISA results showed the specificity of HD5 and HD6 to B272 homodimers when challenged for different recombinant HLA class I complexes (-A1,-B7,-B13,-C7,-B27, and-B272). Control HC10 and W6/32 antibodies were used as positive and negative control, respectively. (B) Direct ELISA against HLA-G and B272 homodimers using HD5, HD6, HC10 and W6/32 antibodies showed specificity of HD5 and HD6 to immobilized B272 homodimers but not to HLA-G homodimers. (C-E) Recombinant B272, B27-free-heavy chain and HLA-B27 heterotrimers were immobilized into chips for kinetic characterization by SPR. C) HD5 and ligand (B272) have a Kd of 0.32 nM. Immobilized free-heavy chains (D) or HLA–B27 heterotrimers (E) did not interact with HD5 and Kd values were not fitted. (F) Blocking competition experiments in SPR were performed by immobilizing B272 to a streptavidin chip followed by injection of HD5 to form B272-HD5 complexes. Injections of LILRB2, KIR3DL2 and Pirb were assessed and binding events recorded.