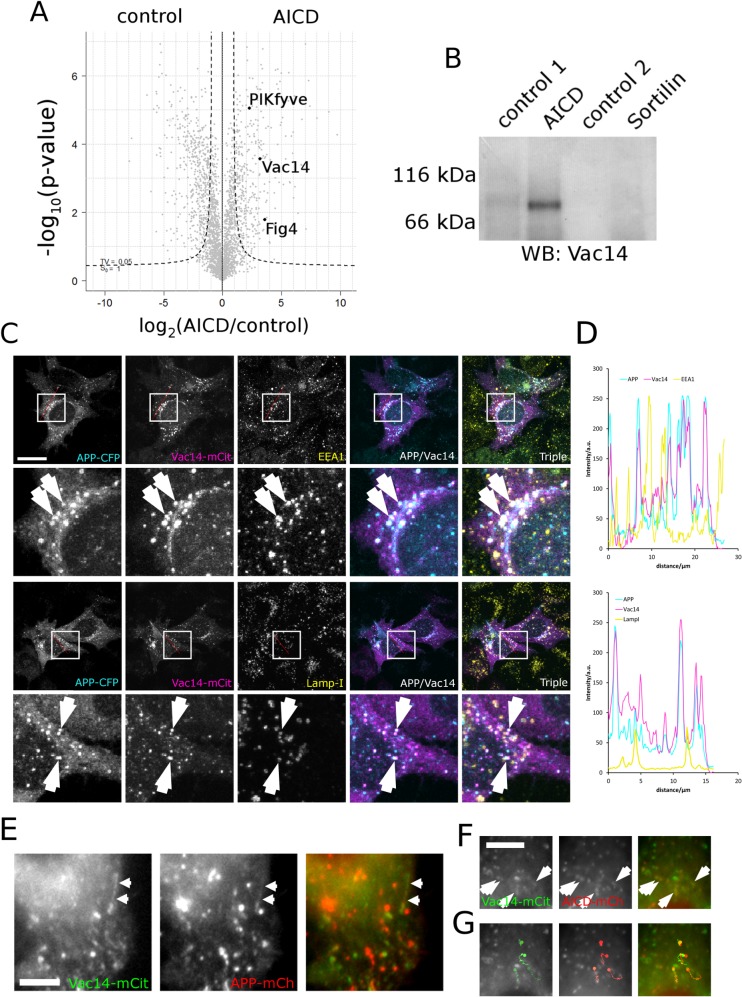
Fig 1. Vac14 associates with the intracellular domain of APP (AICD) biochemically and in HeLa cells.
(A) Proteo-liposome recruitment analysed by mass spectrometry allowed the identification of the intracellular interactome of APP. A Vulcan plot showing that PIKfyve, Vac14 and Fig 4 of the mammalian PIKfyve complex were significantly enriched in AICD proteo-liposomes (right quadrant) compared to controls (left). The dashed line indicates the 0.05 significance threshold. (B) Western blotting confirmed the enrichment of Vac14 by AICD presenting proteo-liposomes while the intracellular domain of the none-related receptor Sortilin and two additional controls (a non-related control peptide designated as 'control 1' or coupled cysteine 'control 2'–both described in [27]) failed to bind Vac14, showing that Vac14 specifically associates with the intracellular domain of APP. (C) APP expressed as a C-terminal CFP fusion displayed strong colocalisation with Vac14-mCit in HeLa cells. Limited colocalisation of APP and Vac14 could be observed on early endosomes (labelled with EEA1) and late endosomes/lysosomes labelled with LampI, as indicated by arrows on the insets. Bar, 20 μm. (D) Line scans (indicated by the red line in (C) demonstrated strong colocalisation between APP and Vac14. (E) In live-cell imaging APP fused to mCherry displayed co-movement with Vac14-mCit positive vesicles and tubular carriers (arrows) that track through the cytoplasm. (F, G) (S1 Video). AICD fused to mCherry also colocalised with Vac14-mCit in live cell imaging (arrows) (S2 Video). (G) Four individual vesicles positive for both AICD and Vac14 were tracked and the first image of the sequence overlayed with the traces to illustrate comigration of AICD and Vac14. Note that in live cell imaging due to the delay caused by the change of filters on fast moving vesicles the staining in the red and green channels are slightly displaced. Bar, 5μm.