Fig 2. Self-reported Fatigue scores for 28 patients receiving rituximab induction and maintenance treatment.
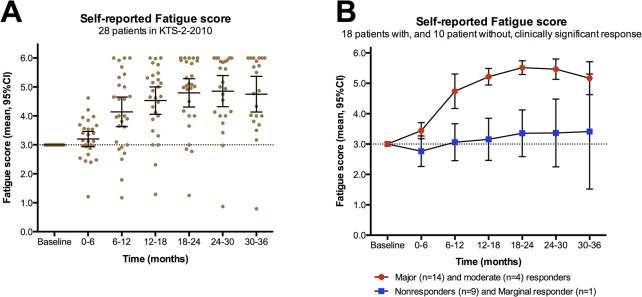
Fatigue score was recorded every second week, always compared to baseline, as the mean of four fatigue-related symptoms (scale 0–6; 3: no change from baseline; 4, 5, 6: slight, moderate, major improvement, respectively; 2, 1, 0: slight, moderate, major worsening, respectively). Panel A shows Fatigue scores for the time intervals 0–6, 6–12, 12–18, 18–24, 24–30 and 30–36 months, with means and 95% CI for each time interval. In panel B the corresponding Fatigue scores are shown for each time interval during follow-up, divided between 18 patients with clinically significant responses, and 10 patients with either marginal response (n = 1) or no response (n = 9). Out of 10 patients with no clinically significant response, one patient withdrew from study after 12 months, and four patients after 24–26 months follow-up. Out of 18 patients with clinically significant responses, one withdrew from study after 24 months due to a diagnosis of T2N0 breast cancer, two moderate responders withdrew after 25 and 32 months, respectively, and one major responder withdrew after 32 months.
