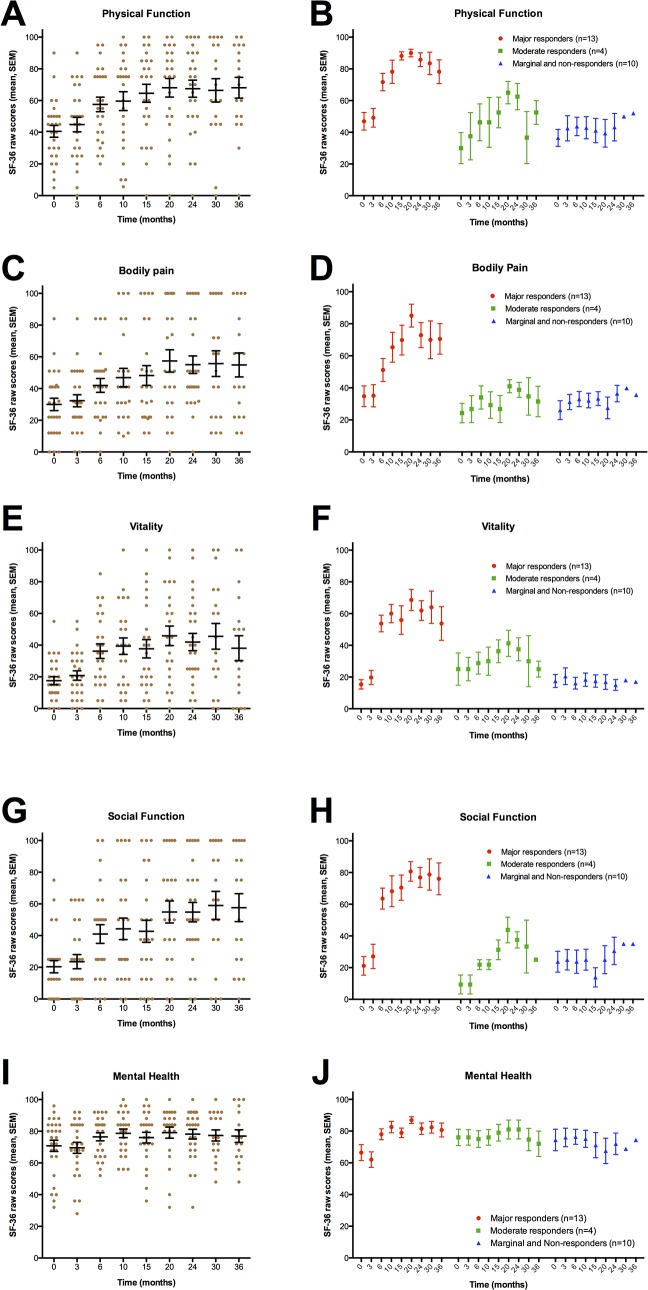
Fig 5. SF-36 questionnaire, raw scores.
SF-36 (Norwegian ver. 1.2) forms were recorded at baseline and at 3, 6, 10, 15, 20, 24, 30 and 36 months. SF-36 raw scores (mean, SEM) are shown for 27 patients, for the subdimensions Physical function (panel A), Bodily pain (panel C), Vitality (panel E), Social function (panel G) and Mental health (panel I). In panels B, D, F, H and J are shown the corresponding SF-36 raw scores separately for 13 major responders, four moderate responders, and 10 patients with no clinical significant response (one marginal responder and nine non-responders). One pilot patient (major responder, withdrew from study after 32 months) did not fill in SF-36 forms. One included patient did not receive induction rituximab infusions due to an allergic reaction to the first infusion, and did not fill in SF-36 forms. One major responder was withdrawn from study after 24 months due to being diagnosed with a T2N0 breast cancer. Out of four moderate responders, one withdrew from the study after 25 months, and one after 32 months. Out of 10 patients with no clinically significant response one withdrew from study after 12 months, and four patients after approximately 24 months follow-up.