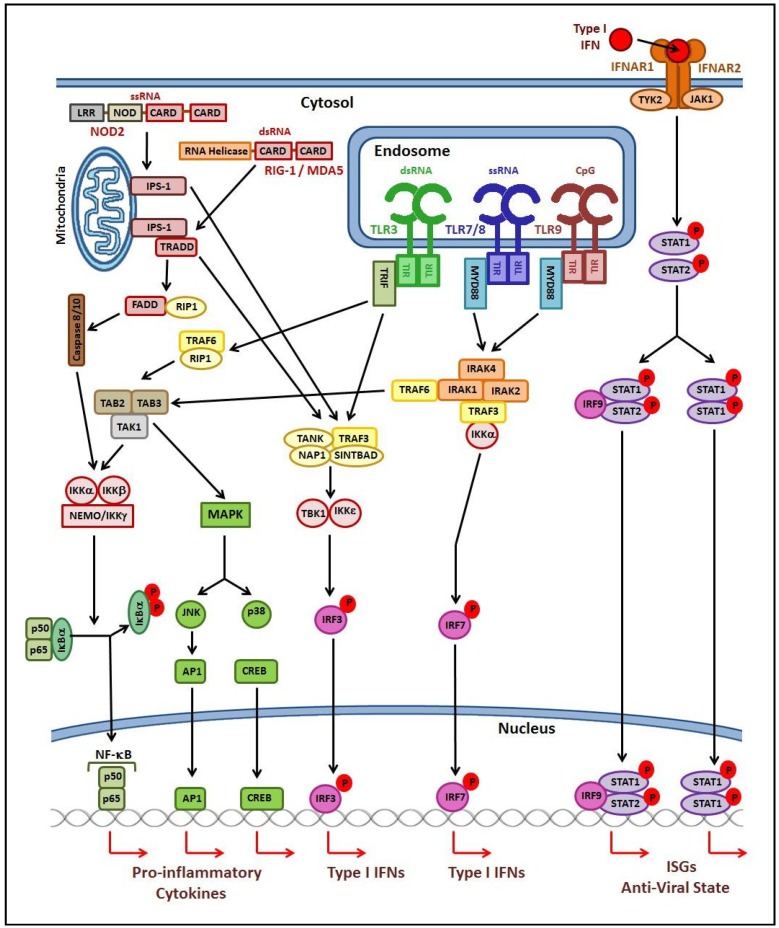
Figure 1.
Pathogen recognition receptor signalling following viral infection. Ligand-induced dimerisation occurs following PAMP recognition by endosomal TLRs, which engages the Toll-IL-1 receptor (TIR) domains to initiate adaptor molecule recruitment and signal transduction. MYD88-dependent signalling results in the formation of an IRAK/TRAF6 complex, which phosphorylates IRF7 to initiate transcription of type I IFN genes, and activates a TAK1/TAB2/3 complex to drive transcription of pro-inflammatory cytokine genes via activation of NF-κB, AP1 and CREB. TRIF-dependent signalling can also activate NF-κB, AP-1 and CREB via recruitment of TRAF6 and RIP1. Alternatively, TRAF3 is recruited, resulting in phosphorylation of IRF3 which translocates into the nucleus to induce expression of type I IFNs. RIG-I and MDA5 are also able to activate NF-κB and IRF3 via interaction with IPS-1 localized on the mitochondrial membrane through homophilic interactions between their CARD domains. Similarly, CARD domains of NOD2 also interact with IPS-1 resulting in transcription of type I IFN genes. The type I IFNs produced bind to their receptor, and, via STAT-mediating signalling, initiate gene transcription.