Abstract
Study design
Cross-sectional cohort study.
Objectives
To investigate a mediational model where pain (intensity and interference) and fatigue mediate the relationship between the use of mobility aids and moderate-to-severe depressive symptomatology among ambulatory participants with spinal cord injury (SCI).
Setting
A medical university in the southeastern United States.
Methods
Ambulatory adults (N = 652) with chronic SCI responded to a mail-in survey. The Patient Health Questionnaire-9 was used to assess moderate-to-severe depressive symptomatology. The Brief Pain Inventory was used to assess pain intensity and interference, and the Modified Fatigue Impact Scale–5-item version was used to assess fatigue. Participants self-reported use of mobility aids.
Results
On examining mobility aids used for ambulation, 65% were found to have used at least one aid. Severe pain intensity was reported by 11%, and 14% reported severe pain interference. Disabling fatigue was reported by 10% of the participants. Twenty-one percent (n = 138) reported moderate-to-severe levels of depressive symptoms. On examining the relationships between mobility aids and depressive symptomatology, using people as a mobility aid was associated with increased odds of depressive symptomatology (2.6) and always using a wheelchair was associated with lower odds (0.3). However, these relationships were no longer significant after controlling for the mediating variables pain intensity, pain interference and fatigue.
Conclusions
Pain and fatigue mediate the relationship between usage of certain mobility aids and depressive symptomatology. The use of people to assist in ambulation is associated with greater odds of moderate-to-severe depressive symptomatology, while always using a wheelchair is associated with lower odds.
Keywords: ambulation, depression, fatigue, pain, rehabilitation, spinal cord injuries
INTRODUCTION
Ambulation is a highly coveted goal after spinal cord injury (SCI). However, functional ambulation after SCI is often compromised by residual impairments, pain, fatigue and reliance on mobility aids, and studies suggest that long-term ambulation may also be associated with negative health outcomes under some circumstances.1–6 Specifically, studies suggest that three associated health conditions, chronic pain, fatigue and depressive symptoms,7 are correlated with ambulatory status.2–7 These secondary health conditions are more prevalent after SCI compared with the general population and negatively impact the quality of life, warranting the need to understand better the relationships of these three outcomes with ambulatory status.
After SCI, overall pain rates range from 25 to 96%, and severe pain is reported by 18–63% of individuals.8–10 Among those with incomplete SCI, pain intensity and interference are associated with the use of mobility aids and degree of independence when walking.1,4,6 A recent study found that higher pain severity is significantly associated with transition from walking to using a wheelchair within the first year after injury.1 Increased odds of high pain intensity are also associated with the use of at least one person for assistance during walking, unilateral cane use and minimal wheelchair usage.3 Furthermore, ambulators are reported to have higher levels of pain interference compared with nonambulators.4 In the incomplete SCI population, higher pain interference scores are associated with needing assistance from another person to walk,2 unilateral cane and wheelchair usage.3 It is important to examine pain intensity and interference among ambulatory individuals with SCI, as pain is associated with a number of negative health outcomes, including fatigue, depressive symptoms and decreased quality of life.2,7–9,11–13
An estimated 67–74% of persons with SCI report fatigue,8,14 though the rates vary according to the level of and time since injury, as well as the definition of fatigue used. Among those with chronic SCI, cross-sectional analyses suggest that severe fatigue is experienced by 18%9 and disabling fatigue by 8.3%.15 Greater fatigue severity has been reported among those with incomplete SCI16 and recently, in an AIS D population, high levels of fatigue were reported by 20%.5 Fatigue can negatively affect the quality of life17 and is associated with aging, injury severity, physiological and psychosocial functioning, spasticity, pain, depression, the use of mobility aids and many behavioral risk factors.3,5,8,15,18–20 Similar to the associations between mobility aid usage and pain observed in an ambulatory chronic SCI population, high levels of fatigue are significantly related to unilateral cane use and wheelchair usage less than 50% of the time.4
Depression has been extensively studied after SCI. The reported prevalence of depressive disorders and symptomatology varies, with ranges from 8.8 to 60%, though most studies report between 20 and 30%.21,22 After SCI, a number of demographic, injury-specific and health-related factors are significantly associated with depressive symptomatology.22 Importantly, among those with incomplete SCI, studies suggest that pain, fatigue and ambulatory status are highly correlated with depressive symptoms.1,2,5 Individuals relying on assistance from others to walk report greater depressive symptoms compared with independent ambulators and wheelchair users; pain interference mediates the observed relationship.2 In those who transition from walking to using a wheelchair, significantly higher depression scores have been reported compared with those who maintain walking or wheelchair use 1 year after injury.1
Two preliminary studies have been conducted using this data set to examine independently the impact of ambulatory status on pain, fatigue and depression, setting the stage for the current study. Pain interference has been reported as a mediator in the relationship between ambulatory status and depressive symptoms after SCI. However, in the original investigation, only a general description of ambulation (independent, partially dependent or nonambulatory) was used, and the effects of specific mobility aids used by ambulatory individuals with SCI were not examined. Furthermore, only pain interference was examined as a mediator.2 In a more recent analysis, the effects of assistive device use on pain intensity, pain interference and fatigue were established. The use of less-supportive assistive devices and wheelchair usage less than 50% of the time was significantly associated with increased pain intensity, pain interference and severe fatigue.23 The present study builds upon the previous findings to examine further the impact of mobility aid usage and the relationships on these significant secondary health outcomes in ambulatory individuals with SCI.
Purpose
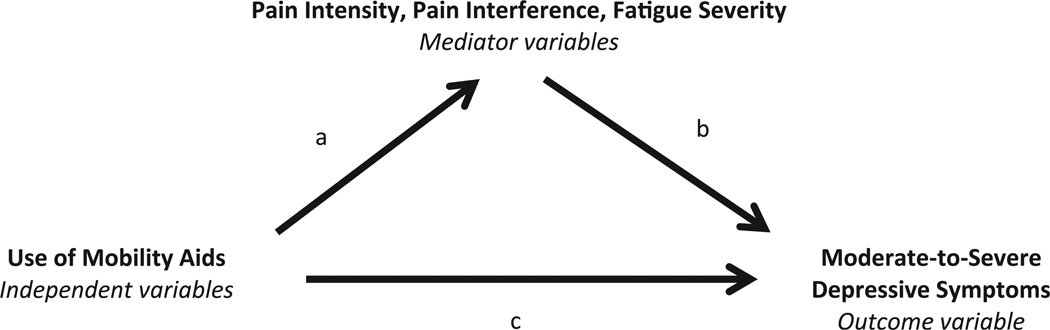
Our purpose was to test a mediational model where pain (intensity, interference) and fatigue mediate the relationship between use of mobility aids and moderate-to-severe depressive symptomatology among ambulatory participants with SCI (Figure 1). Path A has been previously demonstrated and reported using this data set.3 Our hypotheses were threefold: (1) pain intensity, pain interference and fatigue will be significantly correlated with moderate-to-severe depressive symptoms (path B); (2) mobility aid usage will be significantly correlated with depressive symptoms (path C); and (3) after controlling for the relationships between path A and B, the relationship observed in path C will no longer be significant.
Figure 1.
Pain intensity, pain interference and fatigue severity as mediators of the relationship between use of mobility aids for ambulation and depressive symptoms.
MATERIALS AND METHODS
Participants
Following approval from the institutional review board, participants were identified through three sources of records at a large specialty hospital in the southeastern United States: (1) SCI Model Systems database, (2) model systems registry, and (3) outpatient directory. Although participants were identified through one of the SCI Model Systems, our data were specifically collected for this study, and we did not utilize any of the data routinely collected by the SCI Model Systems. Inclusion criteria were: (1) SCI of traumatic origin, (2) at least 1 year post-injury, (3) at least 18 years old at survey, and (4) residual effects of SCI. Seventy-one percent (n = 2614) of the eligible participants responded. After participation, 65 individuals were determined ineligible due to full recovery (n = 16), nontraumatic injury (n = 46) or less than 1 year post injury at survey (n = 3); this resulted in a final sample size of 2549. The current study focused on 783 participants who self-reported the ability to walk.
Procedures
Data were collected by mail-in self-report. Participants responded to a detailed survey packet that has been estimated to take 45–60 min to complete. Potential participants were mailed a preliminary letter detailing the study and informing them that study materials would follow 4–6 weeks later. Those who did not return the initial materials were mailed a second set, and then contacted by phone if they did not respond. If the initial materials were lost or misplaced, a replacement was sent to those who expressed interest in participating. Participants received $50 by way of remuneration.
Measures
Self-report demographic data were collected from the completed instrument packages. Information regarding etiology, time since injury and level of injury (C1–C4, C5–C8, noncervical) was collected, as was ambulation status.
Ambulation status was determined by an initial screening question of ‘Are you able to walk at all?’ (yes, no). Information about mobility aids used to assist in walking was collected, including: walker (yes, no), crutches (none, 1 or 2), canes (none, 1 or 2), short leg braces (none, 1, 2), long leg braces (none, 1, 2) and assistance from people (no, 1 person, 2 people). Lastly, participants reported the amount of time they used a wheelchair to get around, even though they could walk (less than 50%, about 50%, more than 50%, always).
A variable for the total number of mobility aids used was created based on the sum of the following: walker (0, 1), cane(s) (0, 1), crutch(es) (0, 1), short leg brace(s) (0, 1), long leg brace(s) (0, 1) and people (0, 1), where 0 = no and 1 = yes. In addition, walkers, canes and crutches were grouped as ‘assistive devices’ (none, unilateral or bilateral) and short and long leg braces were grouped as ‘leg braces’ (none, 1 short or long leg brace, 2 short or long leg braces).
Pain intensity and interference were assessed based on questions from the Brief Pain Inventory (BPI). The BPI is a valid and reliable measure used to assess pain in the SCI population.24 Pain intensity was determined by four severity items which asked participants to rate their: (1) pain at its worst in the past week, (2) pain at its least in the past week, (3) pain on average, and (4) pain right now, from 0 (no pain) to 10 (worst pain imaginable). The average of the items was used as pain intensity. Participants were also asked to respond to seven items that determined how pain interfered with certain activities in the past week on a 10-point scale (0 = does not interfere, 10 = completely interferes). The average pain interference score was calculated for persons who answered over half of the items.21 Categories for mild (0–3), moderate (4–6) and severe (7–10) pain intensity and interference were created as previously described.4
Fatigue was measured using the Modified Fatigue Impact Scale (MFIS-5), a five-item questionnaire assessing perceived impact of fatigue, defined as a feeling of physical tiredness and lack of energy, over the past 4 weeks. This scale, originally designed for the multiple sclerosis population, has been shown to be valid and reliable.25 Participants responded to each item with a score of 0 (never) to 4 (almost always), and total scores from 0 to 20 were generated. A cutoffpoint ≥15 was used to represent disabling fatigue (yes, no) as previously described.4
Moderate-to-severe depressive symptomatology was measured based on responses to the Patient Health Questionnaire-9 (PHQ-9), which has been frequently used in the SCI population and has good internal consistency, test–retest reliability, and construct and criterion validity.26,27 Participants responded to nine questions with a score of 0 to 3 (0 = not at all, 1 = several days, 2 = more than half of the days, 3 = nearly every day) indicating how frequently they were bothered by a number of problems in the last 2 weeks. The responses were scored and depression severity levels were classified as none (1), minimal (1–4), mild (5–9), moderate (10–14), moderately severe (15–19) and severe (20–27). A cutoff point of PHQ-9 ≥ 10 (yes, no) was chosen for the primary outcome, as individuals with major depression are seven times more likely to have scores ≥10 compared with individuals without major depression.25
Analysis
SAS Version 9.3 was used for all analyses. Descriptive statistics were generated to describe the participant sample, use of mobility aids, pain intensity, pain interference, fatigue and depression severity scores.
The χ2 statistic was used to assess the association between depressive symptomatology and each variable. Variables with a P-value < 0.15 (Table 2) were then put into the first-stage logistic regression model.
Table 2.
Bivariate analyses
| Mobility aids | PHQ-9< 10 No–mild depressive symptoms (n = 514) |
PHQ-9> 10 Moderate–severe depressive symptoms (n = 138) |
χ2 P-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Use any assistance to walk (walker, cane(s), crutch(es), braces, people) | 0.473 | ||||
| Yes | 332 | 78.12 | 93 | 21.88 | |
| No | 178 | 80.54 | 43 | 19.46 | |
| Total number of mobility aids | 0.007a | ||||
| 0 | 182 | 80.18 | 45 | 19.82 | |
| 1 | 166 | 81.77 | 37 | 18.23 | |
| 2 | 115 | 79.31 | 30 | 20.69 | |
| 3 | 45 | 71.43 | 18 | 28.57 | |
| 4+ | 6 | 42.86 | 8 | 57.14 | |
| Walker | 0.425 | ||||
| Yes | 124 | 76.54 | 38 | 23.46 | |
| No | 365 | 79.52 | 94 | 20.48 | |
| Cane | 0.023a | ||||
| Yes | 148 | 73.27 | 54 | 26.73 | |
| No | 358 | 81.18 | 83 | 18.82 | |
| Crutch | 0.815 | ||||
| Yes | 88 | 77.88 | 25 | 22.12 | |
| No | 418 | 78.87 | 112 | 21.13 | |
| Assistive devices (cane(s), crutch(es), or walker) | 0.315 | ||||
| None | 206 | 80.78 | 49 | 19.22 | |
| Unilateral | 154 | 75.49 | 50 | 24.51 | |
| Bilateral | 146 | 80.66 | 35 | 19.34 | |
| Leg braces | 0.111 | ||||
| None | 376 | 79.16 | 99 | 20.84 | |
| 1 short or long leg brace | 65 | 71.43 | 26 | 28.57 | |
| 2 short or long leg braces | 69 | 84.15 | 13 | 15.85 | |
| People | 0.122 | ||||
| Yes | 57 | 72.15 | 22 | 27.85 | |
| No | 456 | 79.72 | 116 | 20.28 | |
| Wheelchair usage (% time) | 0.012a | ||||
| Never to less than 50% | 365 | 78.37 | 98 | 21.63 | |
| About 50% | 14 | 58.33 | 10 | 41.77 | |
| More than 50%, but not always | 47 | 77.05 | 14 | 23.05 | |
| Always | 91 | 87.50 | 13 | 12.50 | |
| Pain intensity | < 0.0001a | ||||
| Mild | 353 | 91.69 | 32 | 8.31 | |
| Moderate | 148 | 69.81 | 64 | 30.78 | |
| Severe | 26 | 37.14 | 44 | 62.86 | |
| Pain interference | < 0.0001a | ||||
| Mild | 410 | 92.76 | 32 | 7.24 | |
| Moderate | 92 | 68.15 | 43 | 31.85 | |
| Severe | 25 | 27.78 | 65 | 72.22 | |
| Fatigue | < 0.0001a | ||||
| Nondisabling | 501 | 85.64 | 84 | 14.36 | |
| Disabling | 13 | 19.4 | 54 | 80.60 | |
If P < 0.15 then the variable was included in the logistic regression models.
Mobility aid usage, pain, and fatigue among ambulatory individuals with SCI and relationships with depressive symptoms.
χ2 P-value < 0.05.
Three separate logistic regression analyses were run to examine the mediational framework. The models were used to predict the odds of having moderate-to-severe depressive symptomatology, classified as a score on the PHQ-9 ≥ 10 (0 = no, 1 = yes). We controlled for age, race, gender, time since injury and injury level. In model 1, predictor variables included cane, leg braces, people and wheelchair usage, as they were the significant variables identified in the χ2 analysis. In model 2, predictors that were no longer significant (cane and leg braces) were removed. Model 3 was the mediational model, and pain intensity, pain interference and fatigue were added. Odds ratios (OR) and 95% confidence intervals (CI) from the logistic models are presented in the results. Odds ratios express the odds of the outcome, depressive symptomatology, for one group of the independent variable compared with the reference group in that variable.
Statement of ethics
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
RESULTS
The cohort was reduced to 652 ambulatory cases with complete data. Table 1 summarizes the participant characteristics. Mean age at enrollment was 46.5 ± 14.4 years old, and participants were on average 10.5 ± 8.2 years post-injury.
Table 1.
Participant characteristics
| % (Unless otherwise indicated) (N = 652) |
|
|---|---|
| Gender | |
| Male | 70.4 |
| Female | 29.6 |
| Race | |
| White | 74.2 |
| Black | 20.5 |
| Other | 5.21 |
| Injury severity | |
| C1–C4 | 25.0 |
| C5–C8 | 27.7 |
| Noncervical | 47.3 |
| Mobility aids | |
| Use any assistance to walk | 65.8 |
| Walker | 26.1 |
| Cane | 31.4 |
| Crutch | 17.6 |
| Long leg braces | 9.8 |
| Short leg braces | 19.3 |
| People | 12.1 |
| Wheelchair usage (% time) | |
| Nevertolessthan 50% | 70.6 |
| About 50% | 3.7 |
| More than 50%, but not always | 9.5 |
| Always | 16.2 |
| Pain intensity (mean (s.d.)) | 3.4 (2.5) |
| Mild | 58.1 |
| Moderate | 31.3 |
| Severe | 13.7 |
| Pain interference (mean (s.d.)) | 3.1 (2.8) |
| Mild | 65.8 |
| Moderate | 20.5 |
| Severe | 13.7 |
| Fatigue (mean (s.d.)) | 7.2 (5.2) |
| Nondisabling | 89.7 |
| Disabling | 10.3 |
| Depressive symptoms (mean (s.d.)) | 6.05 (5.95) |
| No-mild | 78.8 |
| Moderate-severe | 21.2 |
Bivariate results
Based on the χ2 results (Table 2), there was a significant difference in depressive symptomatology among groups according to the total number of mobility aids used (χ2 P-value = 0.0007), cane (χ2 P-value = 0.023) and wheelchair usage (χ2 P-value = 0.012). In addition, there were significant differences observed among pain intensity, pain interference and fatigue groups (χ2 P-value < 0.0001).
Logistic regression
In model 1, those who used people for assistance to walk were 2.48 times more likely to report depressive symptomatology than those who did not use people for assistance (OR = 2.5, 95% CI = 1.25–4.95) (Table 3). Always using a wheelchair was associated with a lower odds (OR = 0.342, 95% CI = 0.15–0.78). In model 2, relationships between people and wheelchair usage and moderate-to-severe depressive symptomatology remained significant. After the addition of the mediators in model 3, severe pain intensity significantly increased the odds of depressive symptoms (OR = 3.32, 95% CI = 1.35–8.19), as did moderate and severe pain interference (OR = 4.65, 95% CI = 2.42–8.94; OR = 10.22, 95% CI = 4.35–24.04) and disabling fatigue (OR = 7.44, 95% CI = 3.38–16.37); the use of people and always using a wheelchair became nonsignificant.
Table 3.
Results from the three stage logistic regression models
| Model 1 | P-value | Model 2 | P-value | Model 3 | P-value | |
|---|---|---|---|---|---|---|
| Injury level (vs noncervical) | 0.422 | 0.407 | 0.213 | |||
| C1–C4 | 0.82 (0.48–1.39) | 0.84 (0.51–1.41) | 1.10 (0.58–2.11) | |||
| C5–C8 | 1.18 (0.73–1.91) | 1.22 (0.77–1.94) | 1.69 (0.93–3.08) | |||
| Race (vs white) | 0.407 | 0.447 | 0.548 | |||
| Non-white | 0.82 (0.51–1.32) | 0.83 (0.52–1.33) | 0.84 (0.46–1.50) | |||
| Gender (vs male) | 0.736 | 0.572 | 0.893 | |||
| Female | 0.93 (0.59–1.45) | 0.88 (0.57–1.37) | 0.96 (0.55–1.69) | |||
| Age | 0.314 | 0.290 | 0.040 | |||
| 0.99 (0.98–1.01) | 0.99 (0.98–1.01) | 0.98 (0.96–0.99) | ||||
| Time since injury | 0.955 | 0.995 | 0.279 | |||
| 0.99 (0.97–1.02 | 1.00 (0.98–1.03) | 0.98 (0.95–1.01) | ||||
| Cane (vs no) | 0.162 | |||||
| Yes | 1.37 (0.88–2.13) | |||||
| Leg braces (vs none) | 0.221 | |||||
| Unilateral | 1.545 (0.90–2.66) | |||||
| Bilateral | 0.861 (0.41–1.83) | |||||
| People (vs no) | 0.009a | 0.006a | 0.608 | |||
| Yes | 2.484 (1.25–4.95) | 2.63 (1.32–5.23) | 1.26 (0.52–3.07) | |||
| Wheelchair usage (vs < 50%) | 0.011a | 0.002a | 0.249 | |||
| About 50% | 2.092 (0.86–5.09) | 2.30 (0.96–5.51) | 1.38 (0.48–4.02) | |||
| More than 50%, but not always | 1.058 (0.52–2.14) | 1.07 (0.56–2.06) | 1.64 (0.75–3.60) | |||
| Always | 0.342 (0.15–0.78) | 0.29 (0.13–0.63) | 0.55 (0.22–1.38) | |||
| Pain intensity (vs mild) | 0.032a | |||||
| Moderate | 1.42 (0.74–2.70) | |||||
| Severe | 3.32 (1.35–8.19) | |||||
| Pain interference (vs mild) | < .0001a | |||||
| Moderate | 4.65 (2.42–8.94) | |||||
| Severe | 10.22 (4.35–24.04) | |||||
| Fatigue (vs nondisabling) | < .0001a | |||||
| Disabling | 7.44 (3.38–16.37) |
Model 1—Base model using the variables with P < 0.15 from Table 1. Predictors of PHQ-9>10, controlling for injury level, race, gender, age and time since injury.
Model 2—Removal of the assistive devices that were not significant predictors of PHQ-9>10, controlling for injury level, race, gender, age and time since injury.
Model 3—Mediating variables pain mean score, pain interference and fatigue added.
Significant; P < 0.05.
DISCUSSION
This study adds to the body of evidence suggesting that long-term ambulation may be associated with negative secondary health outcomes under certain circumstances. The majority (65%) reported using at least one mobility aid during ambulation. The current study extends earlier findings of complications associated with ambulation after SCI by identifying two other potential mediators, pain intensity and fatigue, in the relationship between ambulatory status and depressive symptoms.
Overall, results were consistent with a mediational relationship between mobility aids and severity of depression, with evidence that pain and fatigue functioned as mediators. Hypotheses 1 and 3 were fully supported, as pain intensity, pain interference and fatigue were significantly correlated with depressive symptoms (path B) and, after controlling for the relationships between path A and B, path C was no longer significant. However, the second hypothesis was only partially supported, as not all mobility aids were significantly correlated with depressive symptoms (path C).
It is noteworthy that the prevalence rates found were lower than reported elsewhere in the literature, at least for pain intensity and fatigue.8–10 Depression scores (21% moderate-to-severe levels) were consistent with the existing literature.27 This may relate to the nature of the measures, which were relatively brief screening measures of pain and fatigue, or the unique sample in terms of the number of ambulatory participants.
The results of the present study suggest that pain intensity, pain interference and fatigue should be carefully monitored among long-term ambulators to avoid complications associated with ambulation, particularly depressive symptoms. Given the increase in incomplete injuries, the ongoing devotion to basic science and locomotor rehabilitation research and the longevity of the SCI population, it is likely the number of ambulatory persons with SCI will increase in coming years. Acknowledging the numerous residual impairments, reduced independence and reliance on mobility aids currently observed, it is important to elucidate the circumstances under which negative health outcomes may be associated with walking. In doing so, therapists can tailor interventions to obtain the most beneficial results and monitor the extent to which secondary complications may develop in conjunction with ambulation.
Rehabilitation researchers and professionals may play an important role in further understanding the complications associated with ambulation after SCI. In rehabilitation settings, the goal of achieving maximum functional potential and independence is often associated with the use of less assistance for mobility. However, these findings suggest that therapists ought to consider the potential long-term effects of advocating for reduced wheelchair usage and the introduction of mobility aids offering less support during ambulation for persons with SCI. In addition, the mediational relationship found in this study emphasizes the need for rehabilitation professionals to be aware of the increased risk of depressive symptoms to modify treatment programs and alert appropriate professionals if need be.
Limitations
The present study has a number of limitations. First, all data were self-report, as was necessary due to the large number of participants. Therefore, the self-report was restricted to straightforward information regarding ambulatory status and mobility aids used, in combination with additional psychometric measures with established reliability and validity from the existing literature (for example, fatigue, pain and depression). Second, all data were screen measures. Although this is consistent with larger-scale self-report studies, including the SCI Model Systems national data collection, these measures are limited compared with more detailed clinical measures. Third, the data were cross-sectional; therefore, evaluation of changes was not possible. Fourth, no detailed laboratory measurements were taken to quantify ambulation. Finally, the inclusion criteria were limited to traumatic SCI only; thus, the findings may not be generalizable to individuals with injuries of nontraumatic etiology.
Future directions
Further study is needed to understand better the effects of long-term ambulation on secondary conditions including pain, fatigue and depressive symptoms after traumatic and nontraumatic SCI and the impact of intervention. Examination of these outcomes in locomotor rehabilitation and longitudinal research may provide insight into the impact of change in ambulation over time and the influence on long-term health and quality-of-life outcomes. Furthermore, detailed evaluation of the use of mobility aids in the ambulatory population and associated complications is needed. Such information may influence how rehabilitation researchers and professionals address device use and tailor interventions.
CONCLUSION
The use of people to assist in ambulation after SCI is associated with greater odds of moderate-to-severe depressive symptomatology, while always using a wheelchair is associated with lower odds. These relationships appear to be mediated by pain intensity, pain interference and fatigue. To clarify these relationships, further study of pain, fatigue and depressive symptomatology in ambulatory persons with chronic SCI is warranted.
ACKNOWLEDGEMENTS
The contents of this publication were developed under grants from the Department of Education, NIDRR grant numbers H133G090059 and H133B090005. However, those contents do not necessarily represent the policy of the Department of Education, and you should not assume endorsement by the Federal Government. This publication was supported by the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant Number TL1 TR 000061 and UL1 TR 000062. The authors would like to thank the following persons who contributed to the work reported in the manuscript: Richard Aust, Josh Acuna, Dr Yue Cao, Jennifer Coker, Melinda Jarnecke, Karla Reed and D’Andra Roper.
Footnotes
DATA ARCHIVING
There were no data to deposit.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Riggins MS, Kankipati P, Oyster ML, Cooper RA, Boninger ML. The relationship between quality of life and change in mobility 1 year postinjury in individuals with spinal cord injury. Arch Phys Med Rehabil. 2011;92:1027–1033. doi: 10.1016/j.apmr.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Krause JS, Brotherton S, Morrisette D, Newman S, Karakostas T. Does pain interference mediate the relationship of independence in ambulation with depressive symptoms after spinal cord injury? Rehabil Psychol. 2007;52:162–169. [Google Scholar]
- 3.Saunders LL, Krause JS, DiPiro ND, Kraft S, Brotherton S. Ambulation and complications related to devices after spinal cord injury. J Spinal Cord Med. 2013;36:652–659. doi: 10.1179/2045772312Y.0000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krause JS, Carter RE, Brotherton S. Association of mode of locomotion and independence in locomotion with long-term outcomes after spinal cord injury. J Spinal Cord Med. 2009;32:237–248. doi: 10.1080/10790268.2009.11760778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freixes O, Rivas ME, Agrati PE, Bochkezanian V, Waldman SV, Olmos LE. Fatigue level in spinal cord injury AIS D community ambulatory subjects. Spinal Cord. 2012;50:422–425. doi: 10.1038/sc.2011.175. [DOI] [PubMed] [Google Scholar]
- 6.Krause JS, Morrisette D, Brotherton S, Karakostas T, Apple D. Pain interference in ambulatory spinal cord injury. Top Spinal Cord Injury Rehabil. 2007;12:91–96. [Google Scholar]
- 7.Craig A, Tran Y, Siddall P, Wijesuriya N, Lovas J, Bartrop R, et al. Developing a model of associations between chronic pain, depressive mood, chronic fatigue, and self-efficacy in people with spinal cord injury. J Pain. 2013;14:911–920. doi: 10.1016/j.jpain.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Jensen MP, Kuehn CM, Amtmann D, Cardenas DD. Symptom burden in persons with spinal cord injury. Arch Phys Med Rehabil. 2007;88:638–645. doi: 10.1016/j.apmr.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkers M, Bryce T, Zanca J. Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J Rehabil Res Dev. 2009;46:13–29. [PubMed] [Google Scholar]
- 10.Johnson RL, Gerhart KA, McCray J, Menconi JC, Whiteneck GG. Secondary conditions following spinal cord injury in a population-based sample. Spinal Cord. 1998;36:45–50. doi: 10.1038/sj.sc.3100494. [DOI] [PubMed] [Google Scholar]
- 11.Jensen MP, Hoffman AJ, Cardenas DD. Chronic pain in individuals with spinal cord injury: a survey and longitudinal study. Spinal Cord. 2005;43:704–712. doi: 10.1038/sj.sc.3101777. [DOI] [PubMed] [Google Scholar]
- 12.Ataoglu E, Tiftik T, Kara M, Tunc H, Ersoz M, Akkus S. Effects of chronic pain on quality of life and depression in patients with spinal cord injury. Spinal Cord. 2013;51:23–26. doi: 10.1038/sc.2012.51. [DOI] [PubMed] [Google Scholar]
- 13.Craig A, Tran Y, Middleton J. Psychological morbidity and spinal cord injury: a systematic review. Spinal Cord. 2009;47:108–114. doi: 10.1038/sc.2008.115. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki R, Krahn GL, McCarthy MJ, Adams EJ. Understanding health outcomes: physical secondary conditions in people with spinal cord injury. Rehabil Psychol. 2007;52:338–350. [Google Scholar]
- 15.Saunders LL, Krause JS. Behavioral factors related to fatigue among persons with spinal cord injury. Arch Phys Med Rehabil. 2012;93:313–318. doi: 10.1016/j.apmr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fawkes-Kirby TM, Wheeler MA, Anton HA, Miller WC, Townson AF, Weeks CAO. Clinical correlates of fatigue in spinal cord injury. Spinal Cord. 2008;46:21–25. doi: 10.1038/sj.sc.3102053. [DOI] [PubMed] [Google Scholar]
- 17.Wijesuriya N, Tran Y, Middleton J, Craig A. Impact of fatigue on the health-related quality of life in persons with spinal cord injury. Arch Phys Med Rehabil. 2012;93:319–324. doi: 10.1016/j.apmr.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 18.Barat M, Dehail P, de Seze M. Fatigue after spinal cord injury. Ann Readapt Med Phys. 2006;49:277–282. doi: 10.1016/j.annrmp.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Craig A, Tran Y, Wijesuriya N, Middleton J. Fatigue and tiredness in people with spinal cord injury. J Psychosom Res. 2012;73:205–210. doi: 10.1016/j.jpsychores.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Alschuler KN, Jensen MP, Sullivan-Singh SJ, Borson S, Smith AE, Molton IR. The association of age, pain, and fatigue with physical functioning and depressive symptoms in persons with spinal cord injury. J Spinal Cord Med. 2013;36:483–491. doi: 10.1179/2045772312Y.0000000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Post MW, van Leeuwen CM. Psychosocial issues in spinal cord injury: a review. Spinal Cord. 2012;50:382–389. doi: 10.1038/sc.2011.182. [DOI] [PubMed] [Google Scholar]
- 22.Saunders LL, Krause JS, Focht KL. A longitudinal study of depression in survivors of spinal cord injury. Spinal Cord. 2012;50:72–77. doi: 10.1038/sc.2011.83. [DOI] [PubMed] [Google Scholar]
- 23.Saunders LL, Krause JS, Dipiro ND, Kraft S, Brotherton S. Ambulation and complications related to assistive devices after spinal cord injury. J Spinal Cord Med. 2013 doi: 10.1179/2045772312Y.0000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raichle KA, Osborne TL, Jensen MP, Cardenas D. The reliability and validity of pain interference measures in persons with spinal cord injury. J Pain. 2006;7:179–186. doi: 10.1016/j.jpain.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Amtmann D, Bamer AM, Noonan V, Lang N, Kim J, Cook KF. Comparison of the psychometric properties of two fatigue scales in multiple sclerosis. Rehabil Psychol. 2012;57:159–166. doi: 10.1037/a0027890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bombardier CH, Richards JS, Krause JS, Tulsky D, Tate DG. Symptoms of major depression in people with spinal cord injury: implications for screening. Arch Phys Med Rehabil. 2004;85:1749–1756. doi: 10.1016/j.apmr.2004.07.348. [DOI] [PubMed] [Google Scholar]