Abstract
Purpose
We undertook this pilot prospective cohort investigation to examine the feasibility of functional magnetic resonance imaging (fMRI) assessments in survivors of critical illness and to analyze potential associations between delirium and brain activation patterns observed during a working memory task (N-back) at hospital discharge and 3-month follow-up.
Materials and Methods
At hospital discharge and 3 months later, fMRI assessed subjects' functional activity during an N-back task. Multiple linear regression was used to examine associations between duration of delirium and brain activity, and elastic net regression was used to assess the relationship between brain activation patterns at 3 months and cognitive outcomes at 12 months.
Results
Of 47 patients who underwent fMRI at discharge, 38 (80%) completed the protocol; of 37who underwent fMRI at 3 months, 34 (91%) completed the protocol. At discharge, the mean (SD) percentage of correct responses on the most challenging version (the N2 version) of the N-back task was 70.4 (23.2; range of 20–100) compared with 76 (23.4; range of 33–100) at 3 months. No association was observed between delirium duration in the hospital and brain region activity in any brain region at discharge or 3 months after adjusting for relevant covariates (P values across all 11 brain regions of interest were >.25).
Conclusions
Our data support the feasibility of using fMRI in survivors of critical illness at 3-month follow-up but not at discharge. In this small study, delirium was not associated with distinct or abnormal brain activation patterns, although overall performance on a cognitive task of working memory was poorer than observed in other cohorts of individuals with medically related executive dysfunction, mild cognitive impairment, and mild traumatic brain injury.
Keywords: MRI, Delirium, Cognitive impairment, ICU, Sepsis, Neuroimaging
1. Introduction
Cognitive impairment is pervasive in survivors of critical illness [1–3], but until recently, this population has been studied primarily using neuropsychologic tests. Only a few investigations of this population have used neuroimaging to examine brain structure or function [4]. Several small studies of critically ill cohorts retrospectively examined results of clinical neuroimaging and found a variety of abnormalities including heterogeneous lesions, white matter hyperintensities and diffuse atrophy [5–7]. More recently, prospective investigations using quantitative magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) identified a robust association between delirium duration and generalized brain atrophy and loss of white matter integrity measured by fractional anisotropy in the corpus callosum and internal capsule in survivors of critical illness [8,9].
Of the wide variety of candidate risk factors hypothesized to be associated with long-term cognitive impairment after critical illness, delirium has been particularly widely studied [1]. Investigations of older patients hospitalized outside the intensive care unit (ICU) have assessed neuropsychologic outcomes and consistently found that delirium is associated with an increased risk of cognitive decline or new cognitive impairment [10,11]. We have found similar relationships in critically ill patients [1] and also conducted preliminary investigations (using the same group of patients described herein) that link delirium to brain atrophy and reduced fractional anisotropy [8,9]. Although disrupted neuronal functional connectivity patterns during resting state functional MRI (fMRI) have been identified in non-ICU patients who were actively delirious [12], no ICU-related investigations have used event-related fMRI—a widely used technique for measuring and mapping and defining networks of brain activity during a cognitive task—to assess neural activation after delirium, nor have they evaluated the relationship between fMRI and future cognitive performance, defined via neuropsychologic testing. Importantly, no data exist in survivors of critical illness pertaining to neural activity during cognitive tasks such as the N-back task, which assesses working memory, a key dimension of executive functioning that has been shown to be broadly impaired in survivors of critical illness [13,14].
We sought to determine the feasibility of fMRI in examining cognitive and neural function in survivors of critical illness at discharge and 3-month follow-up. In addition, we hypothesized that longer duration of delirium in the ICU would be associated with subsequent brain activation patterns during the N-back task (a demanding test of working memory) and that brain activation patterns at 3 months would be associated with cognitive functioning at 12 months.
2. Methods
2.1. Study design and population
The VISualizing Icu SurvivOrs Neuroradiological Sequelae (VISIONS) study was a prospective, convenience sample neuroimaging study that was nested within the Bringing to light the Risk Factors And Incidence of Neuropsychological dysfunction in ICU survivors (BRAIN-ICU) study, a prospective cohort study evaluating delirium as a risk factor for long-term cognitive impairment in ICU patients with respiratory failure or shock (detailed inclusion criteria were previously published) [1]. Patients in BRAIN-ICU were eligible for the VISIONS study if they survived to discharge from a medical, surgical, or cardiac ICU at Vanderbilt University Medical Center (Nashville, TN) or Saint Thomas Hospital (Nashville, TN) between June 2006 and December 2009. Exclusion criteria were presence of neurologic disease with known brain lesions, TBI, history of severe dementia or anoxic brain injury, blindness, deafness, non–English speaking, cardiopulmonary bypass within 3 months of ICU admission (to avoid potential bypass-related cognitive impairment), and presence of delirium at hospital discharge. Specific exclusion criteria due to the use of MRI were patient weight greater than 300 lb, claustrophobia, and MRI contraindications (eg, pacemakers or other implanted metal incompatible with MRI).
The institutional review boards at Vanderbilt and Saint Thomas Hospitals approved the study. Because patients were enrolled in the BRAIN-ICU parent study within 72 hours of ICU admission with respiratory failure or shock and often lacked decision-making capacity, written informed consent was obtained from surrogates for collection of demographic and in-hospital data, including delirium evaluation; patients were then asked to provide informed consent prior to MRI imaging once off mechanical ventilation and free of delirium.
2.2. Baseline and demographic characteristics
Baseline clinical and demographic data were collected by study staff at enrollment in the BRAIN-ICU study. Prehospitalization baseline cognitive abilities were evaluated via surrogate interviews that used the validated Short Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE-SF) [15]. The Short IQCODE has strong psychometric properties including good reliability and high sensitivity (75%–100%) and specificity (68%–86%) as a screening test to identify dementia. Patients were suspected to have cognitive impairment if the total Short IQCODE score was higher than 3.3 [15] and were further evaluated with the Clinical Dementia Rating (CDR) scale [16,17]. Patients with a CDR score of 2, reflective of severe preexisting dementia, were excluded. Severity of illness was measured at enrollment using the Acute Physiology and Chronic Health Evaluation II (APACHE II) score [18] and on a daily basis during the ICU stay using the Sequential Organ Failure Assessment (SOFA) score [19]. The MiniMental State Examination was administered to each patient prior to hospital discharge to assess global cognitive function [20].
3. Neuropsychologic measures
As per their participation in the BRAIN-ICU study, patients in the VISIONS ancillary study received neuropsychologic testing at 3- and 12-month follow-up [1] via an evaluation with the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), a well-validated cognitive battery that assesses domains of immediate and delayed memory, attention, visuospatial construction, and language, and produces a total global score that reflects overall performance [21]. They were additionally assessed with Trail Making Test Parts A and B, which measure visual attention and set shifting/executive control, respectively.
3.1. Measures of delirium
Delirium was assessed twice daily in the ICU and once daily thereafter until hospital discharge or up to 30 days after study enrollment by trained research nurses using the Confusion Assessment Method for the ICU (CAM-ICU) [22]. Level of consciousness was measured simultaneously via the Richmond Agitation-Sedation Scale [23]. Delirium duration was defined as the total number of days a patient was delirious according to the CAM-ICU (ie, CAM-ICU positive) during hospitalization (up to 30 days).
3.2. Functional MRI parameters
Magnetic resonance imaging was performed using a Philips Achieva 3.0 Tesla MRI scanner (Philips Healthcare, Inc, Best, the Netherlands) using an 8-channel head coil. Whole-brain 3-dimensional anatomical scans were acquired using a T1-weighted turbo-field echo-pulse sequence. A total of 170 slices were collected with a field of view of 256 × 256 × 170 mm3, repetition time of 8.0 milliseconds, echo time of 3.7 milliseconds, sensitivity encoding factor of 2, flip angle of 5°, and a voxel resolution of 1 mm isotropic. Whole-brain fMRI blood oxygenation level–dependent (BOLD) images were acquired using a T2-weighted echo-planar imaging sequence. A total of 33 slices (3.5 mm with 0.5-mm gap) for 144 volumes were collected in the axial orientation, aligned parallel to the anterior commissure-posterior commissure axis, with a field of view of 240 × 131.5 × 240 mm3, repetition time of 2000 milliseconds, echo time of 35 milliseconds, flip angle of 79, 80 × 80 in-plane acquisition matrix, and final reconstructed voxel size of 1.75 × 1.75 × 4 mm3.
3.3. Functional MRI image processing
The fMRI images were processed with slice timing correction, motion correction, registration to the MNI152 template space (a digital brain template comprised fine anatomical details of the brain), and spatial smoothing. These operations were performed in the SPM5 software (Wellcome Department of Imaging Neuroscience, University College London, London, UK). Each slice's time series was aligned temporally with the inferior-most slice. Then each fMRI volume was rigidly registered with the others from the same volunteer to reduce the effects of head movement. Each volunteer's 3-dimensional image was coregistered with the subject's functional images and then nonlinearly registered to a population template and then to the MNI template using DARTEL, an fMRI data analysis tool [24]. The combined transformation was then applied to the functional images to resample them into MNI space at 3 × 3 × 3-mm voxel size. An 8-mm full-width-at-half-maximum Gaussian spatial smoothing, a tool to quantify image sharpness, was then applied.
3.4. N-back task
We used an fMRI version of a verbal N-back task, a widely used measure of working memory in which a participant views a series of stimuli (in this case letters) and must respond whenever the stimulus presented is the same stimulus that was presented previously.
In this study, we used 0-back (number of intervening items), 1-back, and 2-back conditions. In the 1-back condition, the subject has to remember the position of the letters they had been exposed to ONE turn back. In the 2-back condition, the subject has to remember the position of the letters they have been exposed to TWO turns back. The working memory demand for the 0-back condition is minimal, but the demands on working memory substantially increase in the 2-back condition. The N-back task was presented using EPrime, version 1.2 (http://www.pstnet.com/eprime.cfm). Each test session consisted of 3 blocks each of the 0-back, followed by the 1-back, followed by 2-back (0, 1, 2, 0, 1, 2, 0, 1, 2). Each block consisted of 15 letters presented; 3 letters were targets requiring a response, and 12 letters were foils that required no response. Subjects responded to targets with an index-finger button press. Data were excluded for any subject with less than 2 correct responses per condition (below the “chance” level). Subjects practiced the task prior to entering the scanner, and each participant attempted 2 test sessions while in the scanner.
3.5. Statistical analyses
Descriptive statistics are presented using medians and interquartile ranges for continuous variables and proportions for categorical variables. We primarily describe feasibility by the percent of fMRI-scanned patients who were able to successfully complete the N-back task with better than chance performance and absence of movement artifact on neuroimaging that precluded image analysis.
At the individual level, each N-Back block was modeled in a general linear regression and contrasted for the comparison of 2-back > 0-back. At the group level, a 1-sample t test was performed to determine the overall activation patterns and find the regions of interest (ROIs) for the fMRI data at hospital discharge, potentially enabling us to identify patterns of activity across an overall map. An 8-mm sphere around the local maxima at P = .001 (uncorrected) was used to determine the ROIs, focusing the selection on 11 brain regions that are known to have increased or decreased BOLD response during the N-back task [25].
To examine associations between duration of delirium and regional brain activation, we used multiple linear regression models with duration of delirium as the primary exposure variable and activation in each separate brain region as the outcome, adjusted for the following covariates: (a) age at study enrollment, (b) presence of sepsis at any time during the ICU stay, and (c) percent of correct responses to targets on the fMRI N-back test. These covariates were selected on an a priori basis. Age and sepsis are variables well known to influence cognition and thus were believed to be important to adjust for [14]. We adjusted for percentage of correct responses on the fMRI N-back test, as we believed that it was possible that individuals with more errors could display associated changes in their fMRI signals.
Each regression coefficient estimates the difference in activation of the ROI between patients with delirium duration at the 75th vs the 25th percentile of our population.
In order to evaluate the association between fMRI activation patterns at 3 months and cognitive outcomes (RBANS global score and Trails-B t score) at 12 months, we used a method known as elastic net regression [26]—an approach that is thought to be superior to simple linear regression when there are a large number of predictors (in our case, the more than 18,000 voxels in the brain, each with its own measurement of N-back BOLD response) and a substantially smaller number of patients. This method has been used in fMRI-related investigations previously [27,28] and was conducted using the Matlab statistical package (The MathWorks, Inc., Natick, MA).
Given the hypothesis-generating nature of this pilot study, we did not adjust for multiple comparisons, preferring to examine all possible associations in order to inform future research.
R version 2.13 was used for these analyses [29]. In reporting results of linear regression for continuous variables, we compared the 75th percentile value of the independent variable of interest of our population to the 25th percentile, to provide a more clinically meaningful comparison.
4. Results
4.1. Baseline demographics
Of the 335 patients screened between June of 2007 and December of 2009, 10 declined participation, 142 met MRI exclusions, 20 died before hospital discharge, 60 were actively delirious at hospital discharge, and 41were discharged prior to being consented. The 62 remaining patients were enrolled in VISIONS (Table 1). Patients were severely ill at ICU admission, with a median APACHE II score of 24 (interquartile range, 18–29) and a median SOFA score of 9 [7,12]. The patients' median age was 58 (48–65) years (see Table 1). Only 2 patients had preexisting cognitive impairment per Short ICQODE assessment. These 2 patients were then further assessed for cognitive impairment using the CDR; both had mild cognitive impairment (MCI; CDR 0.5) and were not excluded [17]. Delirium occurred in 70% of patients during their critical illness, with more than 25% of patients experiencing delirium for 3 or more days. No patients were delirious at the time of imaging.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic | Cohort (n = 47) |
|---|---|
| Age at enrollment (y) | 58 (48, 65) |
| Female, % (n) | 38 (18) |
| Education (y) | 12 (12, 14) |
| Short IQCODE | 3 (3, 3.06) |
| Baseline cognitive impairment, % (n/total)a | 4 (2) |
| APACHE II at enrollment | 24 (18, 29) |
| SOFA score at enrollment | 9 (7, 12) |
| Mechanical ventilation, % (n) | 94 (44) |
| Sepsis during ICU stay, % (n) | 57 (27) |
| Admission diagnoses, % (n) | |
| Sepsis/Acute respiratory distress syndrome | 30 (14) |
| Hepatobiliary/Pancreatic surgery | 21 (11) |
| CHF/MI/Cardiogenic shock | 13 (6) |
| ARDS without infection | 9 (4) |
| COPD/Asthma | 6 (3) |
| Vascular surgery | 4 (2) |
| Other diagnoses | 17 (8) |
| ICU length of stay (d) | 4 (2, 6) |
| Hospital length of stay (d) | 9 (6, 13) |
| Delirium prevalence, % (n/total) | 70 (32) |
| Delirium duration (d) | 1 (0, 3) |
| Coma duration (d) | 1 (0, 2) |
CHF indicates congestive heart failure; MI, myocardial infarction; ARDS, acute respiratory distress syndrome; COPD, chronic obstructive pulmonary disease.
Baseline cognitive impairment was defined as having a score higher than 3.3 on the IQCODE-SF. Data are presented as medians and interquartile ranges.
4.2. Qualitative findings
Of the 62 patients enrolled into VISIONS, 12 experienced either physical or psychological distress in the MRI scanner prior to the initiation of the VISIONS protocol (which consisted of structural MRI, DTI, and fMRI in that order) and another 3withdrewprior to the initiation of the fMRI protocol and after the completion of structural MRI and DTI, resulting in a total of 47 patients who completed fMRI and the N-back at discharge. Of these, 1 had poor quality imaging, 5 failed to perform the task, and 3 had both poor quality imaging due to movement and failed to perform the task, resulting in a total of 38 patients with usable fMRI data at discharge. Overall, feasibility of fMRI at discharge was therefore demonstrated in 38 (80%) of 47 patients in whom fMRI imaging was initiated. If feasibility was determined using the entire VISIONS sample (n=62) as the denominator, then feasibility was demonstrated in 61% of patients.
At 3-month follow-up, 10 patients did not receive an fMRI of the 47 who performed the fMRI protocol at discharge: 3 patients died, 1moved out of the area, 3 were not scanned due to chronic health conditions, 1 was claustrophobic, and 2 declined follow-up fMRI scans. Of the 37 patients scanned, 1 had poor quality imaging, 1 failed to perform the task, and 1 had both poor quality imaging due to movement and failed to perform the task, resulting in 34 patients with usable fMRI data at 3-month follow-up. Overall, feasibility of fMRI at 3-month follow-up was therefore demonstrated in 34 (91%) of 37 patients in whom fMRI scanning was initiated. If feasibility was determined using the entire VISIONS sample of patients who were still alive and available at 3 months (n = 58, reflecting all individuals still alive and in the area), then feasibility was demonstrated in 58% of patients.
5. N-back test results
Among the 47 patients who initiated the fMRI protocol at hospital discharge, a total of 9 patients (19%) were unable to complete the N-back at discharge, in many cases likely reflecting severe cognitive impairment and physical debility in the early ICU recovery period. In contrast, at 3-month follow-up, only 3 (8%) of the 34 patients who began the fMRI scanning process were unable to complete the N-back test. No statistically significant difference in N-back performance between discharge and 3 months was observed (P = .24). At discharge, the mean (SD) percentage of correct responses on the N2 task was 70.4 (23.2; range of 20–100). At 3-month follow-up, the mean (SD) percentage of correct responses on the N2 task was 76 (23.4; range of 33–100).
6. Delirium and brain regional activations
No statistically significant associations were observed between delirium duration in the ICU and any activation in any brain region at discharge or 3 months, after adjusting for relevant covariates (P values across all 11 brain ROIs were ≥.25; Table 2).
Table 2.
| Discharge (n = 38) | 3 mo (n = 34) | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | Lower | Upper | P | Coefficient | Lower | Upper | P | |
| Increased activation | ||||||||
| Left inferior parietal | −0.0092 | −0.0535 | 0.0350 | .6847 | 0.0050 | −0.0339 | 0.0438 | .8034 |
| Right inferior parietal | −0.0014 | −0.0256 | 0.0227 | .9075 | 0.0239 | −0.0417 | 0.1003 | .4249 |
| Left precentral | −0.0031 | −0.0526 | 0.0463 | .9016 | 0.0083 | −0.0456 | 0.0622 | .7648 |
| Right middle frontal | −0.0079 | −0.0411 | 0.0252 | .6419 | 0.0155 | −0.0498 | 0.0808 | .6447 |
| Left superior parietal | 0.0052 | −0.0364 | 0.0260 | .7471 | −0.0011 | −0.0529 | 0.0507 | .9662 |
| Right superior occipital | −0.0019 | −0.0238 | 0.0200 | .8674 | 0.0086 | −0.0408 | 0.0580 | .7361 |
| Left middle frontal | −0.0153 | −0.0585 | 0.0879 | .4923 | 0.0018 | −0.0723 | 0.0758 | .9630 |
| Right middle frontal | −0.0060 | −0.0294 | 0.0173 | .6165 | −0.0017 | −0.0484 | 0.0450 | .9431 |
| Left supplementary motor area | −0.0043 | −0.0335 | 0.0250 | .7767 | 0.0090 | −0.0486 | 0.0665 | .7616 |
| Decreased activation | ||||||||
| Superior medial frontal gyrus | 0.0002 | 0.0441 | 0.0444 | .9934 | −0.0051 | −0.0900 | 0.8799 | .9075 |
| Left posterior cingulate | 0.0024 | −0.0274 | 0.0321 | .8774 | 0.0300 | −0.0206 | 0.0806 | .2540 |
Coefficients represent a comparison from the 25th to 75th percentiles of delirium duration (from 0 to 3 days of delirium).
All models are adjusted for age at study enrollment, presence of sepsis at any time during the ICU stay, percent of correct responses to targets on the fMRI N-back test, and mean gray matter volume fraction in the corresponding region.
7. Activation patterns
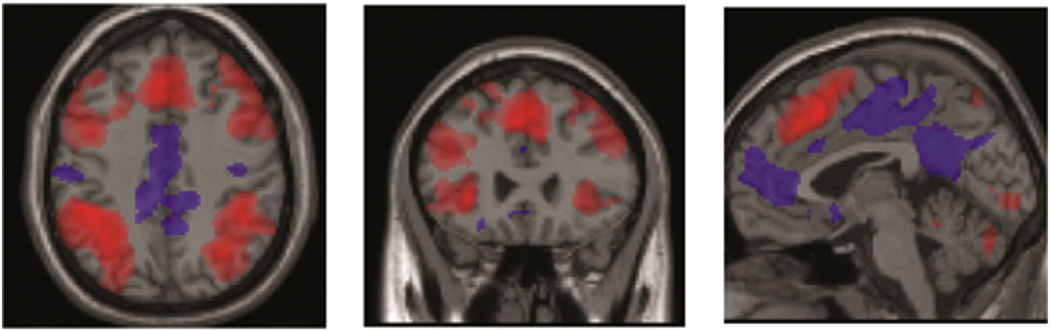
We found that fMRI activation was increased for the 2-back task compared with the 0-back task in 11 brain regions (Table 3) in the frontoparietal network and medial brain after correcting for multiple comparisons (P < .05, family-wise error [FWE]) consistent with numerous previous studies [30], showing that the task engaged the desired executive control and working memory systems of the brain (shown in red in Fig. 1). Decreased activation in medial brain regions (shown in blue in Fig. 1) is consistent with many previous findings of normally decreased activity in the default mode network during attention-demanding tasks [31].
Table 3.
Activation patterns by ROI analysis
| Region | Coordinate |
|---|---|
| Increased activation | |
| Left inferior parietal | (−42, −40, 40) |
| Right inferior parietal | (44, −40, 46) |
| Left precentral | (−44, 4, 50) |
| Right middle frontal | (34, 6, 60) |
| Left superior parietal | (−32, −62, 46) |
| Right superior occipital | (32, −64, 40) |
| Left middle frontal | (−42, 32, 32) |
| Right middle frontal | (36, 38, 28) |
| Left supplementary motor area | (−6, 20, 52) |
| Decreased activation | |
| Superior medial frontal gyrus | (0, 60, 12) |
| Left posterior cingulate | (−2, −44, 30) |
The data presented above describes brain regions (coordinates refer to standard geographic locations of individual brain regions using Montreal Neurologic Institute definitions) marked by increased or decreased activation during the 2-back task compared to the 0-back task. Findings demonstrate the prominence of increased brain activation, occurring largely in the prefrontal and parietal regions—regions known to be implicated in working memory. Listed regions were activated at P<0.05, using a family-wise error correction method that is standard in neuroimaging analyses to account for multiple comparisons—1 t test per voxel—by factoring in the number of voxels and the image smoothness.
Fig. 1.
Brain activation patterns presented in aggregate from the study population at hospital discharge by axial, coronal, and sagittal views, with increased activation shown in red and decreased activation shown in blue. These images show primary patterns of increased activation which occur largely in frontal and parietal brain structures and, less predominantly, patterns of decreased activation which occur largely in posterior cingulate and presupplementary motor cortices.
8. Association between brain activation patterns and long-term cognitive functioning
Functional MRI activation patterns at 3-month follow-up did not predict cognitive outcomes on RBANS or Trails B at 12 months using elastic net regression. Table 4 shows the root mean squared prediction error in units of the cognitive test scores (eg, Trails B score) for a null model and for a model containing the fMRI predictors. The null model assigns the same predicted score to every patient.
Table 4.
fMRI data at 3 Months vs cognitive outcomes at 12 months: elastic net regression results
| Outcome | Null model prediction error | FMRI prediction error |
|---|---|---|
| Trails B | 14.7 | 14.6 (95% CI, 14.4–14.8) |
| RBANS | 15.5 | 15.5 (95% CI, 15.3–15.6) |
Elastic net is a variant of linear regression that allows principled use of many more predictors than observations, as is the case here where we predict outcomes in patients using fMRI signals from approximately 30 000 brain voxels. Functional MRI images acquired at 3-month follow-up did not predict cognitive outcomes on RBANS and Trails B tasks at 12 months. The null model (which assumes all patients had the same outcome) predicted, for example, 12 month Trails B with an error of 14.7 points. Adding fMRI data to the linear model did not significantly improve the prediction of Trails B compared with the null model (error of 14.6 points).
CI indicates confidence interval.
9. Discussion
The primary purpose of our study was to determine the feasibility of using fMRI to study neurologic function of survivors of critical illness at hospital discharge and 3 months later. We found that fMRI was difficult for many patients who attempted it in the immediate posthospital period from both a cognitive perspective—performance was below chance and worse than in neurologically compromised medical populations and in individuals with MCI [32] and mild traumatic brain injury (MTBI) [33] (Fig. 2)—and due to the physical demands of imaging (patients were unable to remain still resulting in significant movement artifact). Although these difficulties persisted at 3 months postdischarge, this period was generally more successful and may represent a more appropriate time to assess fMRI in future studies. This study adds to the small but growing body of research using diverse neuroimaging modalities with individuals after critical illness and lays the groundwork for larger and more definitive investigations using fMRI.
Fig. 2.
Percent of accurate responses (of 100%) on the 2-back condition of the N-back test across clinical populations, with a red horizontal line reflecting the performance of normal controls (mean, 88) from an external non-Vanderbilt data set. Data portrayed here are derived from the current study (in the case of critically ill patients) or from published literature (all other populations). In obtaining data from published literature, we sought—whenever possible—to choose patients broadly similar to our cohort with regard to age, in particular, and to use data that were broadly representative of relevant literature at large. Means and SDs across populations are as follows: CI: 76 (23.4), PD: 77 (7.7), MS: 83.47 (14.8), MCI: 81 (4), MTBI: 83 (7.0), and AD: 55 (4). CI indicates critically ill; PD, Parkinson disease; MS, multiple sclerosis; AD, Alzheimer disease.
As various researchers have documented, cognitive impairment around the time of hospital discharge is often severe and debilitating [34,35]. Jones and colleagues [13] demonstrated that 100% of nondelirious ICU survivors had cognitive impairment at hospital discharge, with 85% having cognitive impairment 1 week later. Cognitive impairment was likely a partial or primary driver of the inability of our cohort to engage in fMRI. Although cognitive impairments and physical limitations persisted at 3 months in many patients, others had dramatic improvements in both areas at this period, which together may have combined to enable a nonsignificantly higher percentage of participants to engage successfully the N-back task at 3-month follow-up.
Although cognitive impairment in survivors of critical illness tends to be diffuse, evidence from numerous cohort studies highlights the prominence of deficits that pertain broadly to executive functioning, including difficulties with aspects of attention, planning, fluency (ie, the ability to rapidly generate novel responses), and set shifting [14]. The impaired performance of our cohort on the N-back task, a putative measure of working memory [36]—a key dimension of executive functioning [37]—underscores these findings. Indeed, our patients display poorer working memory ability on the N-back task than has been observed in other medical populations with well-described and primary frontal lobe deficits, including multiple sclerosis and Parkinson disease [36], as well as among individuals with MTBI [33] and MCI [32], both conditions that are often marked by executive dysfunction. Alternatively, the working memory abilities in survivors of critical illness at 3-month follow-up are significantly better than those observed in elderly individuals with Alzheimer disease [32] (Fig. 2).
Although primarily about feasibility, a secondary goal of our study involved evaluating both the potential relationship between delirium in the hospital and abnormal brain activation patterns during an fMRI-related working memory task (N-back) and the potential relationship between brain activation patterns at 3 months and cognitive outcomes at 12 months. Although delirium has been consistently associated with cognitive impairment at distal time points across modalities including neuropsychologic testing [1], self-report questionnaires [2], and MRI and DTI (in the VISIONS cohort) [8,9], we found no association between delirium duration and N-back performance. This negative finding could reflect the truth, could reflect a type II error (due to our small sample size), could reflect the fact that fMRI is less sensitive to pathology than other imaging modalities, or could suggest that delirium is not associated with deficits in the specific dimensions of working memory assessed by the N-back, even as evidence has demonstrated a robust relationship between delirium and other aspects of executive functioning such as set shifting. In a related vein, the absence of an association between brain activation patterns observed during an N-back task at 3 months and cognitive functioning at 12-month follow-up may largely be due to the fact that the cognitive testing battery we used did not include any direct or indirect working memory measures.
As in any feasibility study, our investigation has several limitations worthy of mention. Our study was done with a relatively small sample and it used a single task—the N-back task, which, while targeting working memory, does not evaluate other important elements of executive functioning that have been shown to be impaired after critical illness. We also did not use a control group, which would have allowed us to compare our study population with another reference population. The absence of a control group, in particular, prevented us from discerning whether there may have been distinctly different patterns in survivors of critical illness vs healthy controls both with regard to activation patterns and the magnitude of change within the task and over time (eg, N1 vs N2, discharge vs 3-month follow-up). Lastly, we want to point to the variations in the manner of defining “feasibility.” We chose to define feasibility in the context of how effectively individuals could engage the fMRI protocol among those who attempted the protocol (eg, among those survivors who underwent an fMRI). Others may have different definitions of feasibility that pivot on broader definitions of “eligibility” than we used (eg, among all ICU patients). Using these broader definitions of feasibility could lead to the conclusion that fMRI in recent survivors of critical illness is less feasible than it appears to be using our chosen definition. This is particularly true at the time of hospital discharge, where nearly 40% of patients were physically or psychologically debilitated to a degree that prevented them from participating in fMRI imaging.
10. Conclusions
Neuroimaging is an increasingly important component of investigations of cognitive impairment among survivors of critical illness. Functional fMRI is unique in its ability to identify brain activation patterns, and these patterns have not yet been elucidated in large investigations of critically ill survivors. Our data support the feasibility of using fMRI in survivors of critical illness at 3-month follow-up but not at discharge—as such, future studies should cautiously integrate fMRI at this time point, if they choose to use such an imaging modality. In this small study, delirium was not associated with distinct or abnormal brain activation patterns, although overall performance on a cognitive task of working memory was poorer than observed in other cohorts of individuals with medically related executive dysfunction, MCI, and MTBI. Integrating a wider array of fMRI tasks, in particular, may provide a fuller understanding of the link between delirium and brain activation patterns, as delirium's impact may be variably expressed across brain regions.
Footnotes
Funding acknowledgment: National Institutes of Health (AG027472, AG034257, RR024975, EB001628, TR000445); Saint Thomas Foundation (Nashville, TN); Veterans Affairs Tennessee Valley Geriatric Research, Education, and Clinical Center; Vanderbilt Institute of Imaging Science; and the Vanderbilt Institute of Clinical and Translational Research.
References
- 1.Pandharipande PP, Girard TD, Jackson JC, Morandi JL, Thompson BT, Pun NE, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson JC, Hart RP, Gordon SM, Shintani A, Truman B, May L, et al. Six-month neuropsychological outcome of medical intensive care unit patients. Crit Care Med. 2003;31(4):1226–1234. doi: 10.1097/01.CCM.0000059996.30263.94. [DOI] [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–1794. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hopkins RO, Jackson JC. Neuroimaging after critical illness: implications for neurorehabilitation outcome. NeuroRehabilitation. 2012;31(3):311–318. doi: 10.3233/NRE-2012-0798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins RO, Gale SD, Weaver LK. Brain atrophy and cognitive impairment in survivors of acute respiratory distress syndrome. Brain Inj. 2006;20(3):263–271. doi: 10.1080/02699050500488199. [DOI] [PubMed] [Google Scholar]
- 6.Morandi A, Gunther ML, Vasilevskis EE, Girard TD, Hopkins RO, Jackson JC, et al. Neuroimaging in delirious intensive care unit patients: a preliminary case series report. Psychiatry. 2010;7(9):28–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Suchyta MR, Jephson A, Hopkins RO. Neurologic changes during critical illness: brain imaging findings and neurobehavioral outcomes. Brain Imaging Behav. 2010;4(1):22–34. doi: 10.1007/s11682-009-9082-3. [DOI] [PubMed] [Google Scholar]
- 8.Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2022–2032. doi: 10.1097/CCM.0b013e318250acc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morandi A, Rogers BP, Gunther ML, Merkle K, Pandhairpande P, Girard TD, et al. The relationship between delirium duration, white matter integrity, and cognitive impairment in intensive care unit survivors as determined by diffusion tensor imaging: the VISIONS prospective cohort magnetic resonance imaging study*. Crit Care Med. 2012;40(7):2182–2189. doi: 10.1097/CCM.0b013e318250acdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2012;21(1):30–42. doi: 10.1080/09540260802675031. [DOI] [PubMed] [Google Scholar]
- 11.Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- 12.Choi SH, Lee H, Chung TS, Park KM, Jung YC, Kim SI, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatr. 2012;169(5):498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- 13.Jones C, Griffiths RD, Slater T, Benjamin KS, Wilson S. Significant cognitive dysfunction in non-delirious patients identified during and persisting following critical illness. Intensive Care Med. 2006;32(6):923–926. doi: 10.1007/s00134-006-0112-y. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox ME, Brummel NE, Archer K, Ely EW, Jackson JC, Hopkins RO. Cognitive dysfunction in ICU patients: risk factors, predictors, and rehabilitation interventions. Crit Care Med. 2013;41(9) Suppl. 1:S81–S98. doi: 10.1097/CCM.0b013e3182a16946. [DOI] [PubMed] [Google Scholar]
- 15.Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 16.Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 17.Berg L. Clinical Dementia Rating (CDR) Psychopharmacol Bull. 1988;24(4):637–639. [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 19.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) manual. San Antonio: Psychological Corporation; 1998. [Google Scholar]
- 22.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 23.Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O'Neal PV, Keane KA, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 24.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B. 2005;67(2):301–320. [Google Scholar]
- 27.Sommer JC, Gertheiss J, Schmid VJ. Spatially regularized estimation for the analysis of dynamic contrast-enhanced magnetic resonance imaging data. Stat Med. 2014;33(6):1029–1041. doi: 10.1002/sim.5997. [DOI] [PubMed] [Google Scholar]
- 28.Shen L, Sungeun K, Yuan Q, Inlow M, Swaminathan S, Nho K, et al. Identifying neuroimaging and proteomic biomarkers for MCI and AD via the elastic net. 2011:27–34. doi: 10.1007/978-3-642-24446-9_4. NIH-PA Author Manuscript, 7012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5(3):299–314. [Google Scholar]
- 30.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buckner RL. The serendipitous discovery of the brain's default network. NeuroImage. 2012;62(2):1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Rombouts SA, Barkhof F, Goekoop R, Stam CJ, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer's disease: an fMRI study. Hum Brain Mapp. 2005;26(4):231–239. doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen CJ, Wu CH, Liao YP, Hsu HL, Tseng YC, Liu HL, et al. Working memory in patients with mild traumatic brain injury: functional MR imaging analysis. Radiology. 2012;264(3):844–851. doi: 10.1148/radiol.12112154. [DOI] [PubMed] [Google Scholar]
- 34.Hopkins RO, Weaver LK, Pope D, Orme JF, Bigler ED, Larson-Lohr V. Neuropsychological sequelae and impaired health status in survivors of severe acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;160(1):50–56. doi: 10.1164/ajrccm.160.1.9708059. [DOI] [PubMed] [Google Scholar]
- 35.Woon FL, Dunn CB, Hopkins RO. Predicting cognitive sequelae in survivors of critical illness with cognitive screening tests. Am J Respir Crit Care Med. 2012;186(4):333–340. doi: 10.1164/rccm.201112-2261OC. [DOI] [PubMed] [Google Scholar]
- 36.Miller KM, Price CC, Okun MS, Montijo H, Bowers D. Is the n-back task a valid neuropsychological measure for assessing working memory? Arch Clin Neuropsychol. 2009;24(7):711–717. doi: 10.1093/arclin/acp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nee DE, Brown JW, Askren MK, Berman MG, Demiralp E, Krawitz A, et al. A meta-analysis of executive components of working memory. Cereb Cortex. 2013;23(2):264–282. doi: 10.1093/cercor/bhs007. [DOI] [PMC free article] [PubMed] [Google Scholar]