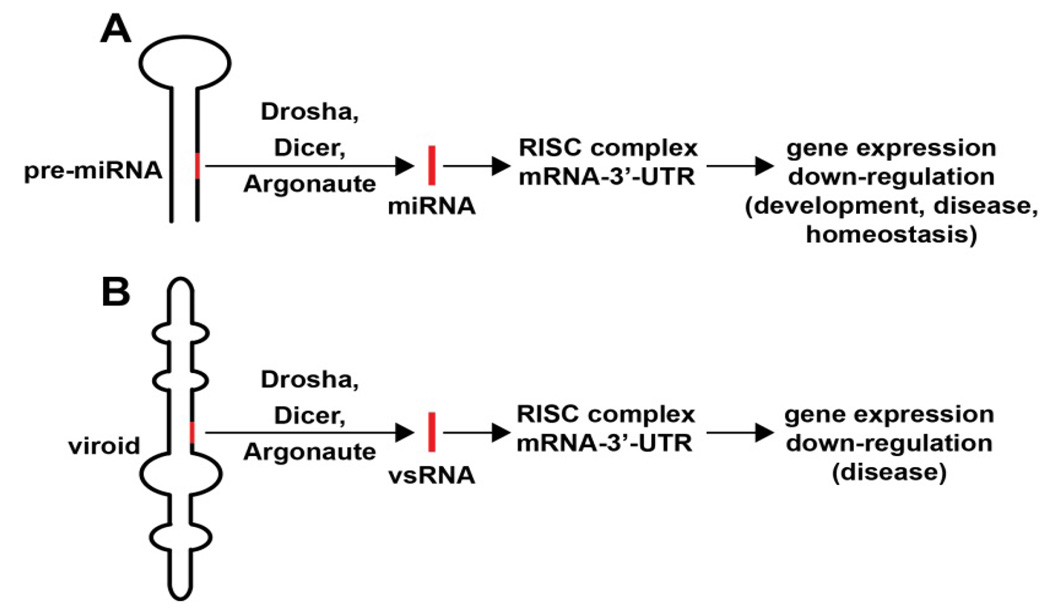
Figure 1. Similarities in miRNA and viroid structure and function.
This highly schematicized figure underscores the remarkable similarities between the structure and function of miRNA and viroids; (A) a typical 75–110 nucleotide primary micro RNA (pri-miRNA) ‘hairpin’ containing an endogenous 21–25 nt miRNA that yields a mature miRNA (red bar) after Drosha- and Dicer-mediated excision and processing; the mature miRNA next associates with a cytoplasmic RNA-induced silencing complex (RISC) and a target mRNA-3’-UTR to degrade and down-regulate expression of that target mRNA, with subsequent effects on the expression of genes involved in homeostasis, development and disease; (B) analogously, a ~246–401 nucleotide closed circular viroid, also containing extensive intra-strand base pairings, dsRNA and stem-loop structures, typically contains a 21–25 nucleotide viroid-specific RNA (vsRNA; red bar) that after host Drosha/Dicer-based processing yields a mature vsRNA; as is the case for miRNAs this vsRNA subsequently targets the RISC and mRNA-3’-UTR complex, down-regulating gene expression to induce progressive developmental and age-related disease in plants [26,27,40,44,72]. In both cases larger miRNA or vsRNA precursors are processed by an RNase III of the family of Dicer-like proteins to generate smaller ‘infectious’ ssRNA species; these sizes are similar to endogenous small interfering RNA (as mature vsRNA or miRNA) to alter the viroid-dependent gene expression in the host plant by viroids, or of miRNA-mRNA processing in animal species including humans [14,26,27,37,42,44,72]. While naked, mature RNAs such as miRNAs and vsRNAs have relatively short half-lives in vitro (for example human neuronal miRNAs appear to be highly labile [27,43], stabilities may be greatly extended by single- or double-stranded RNA-binding proteins, by complex secondary structures, by RNA circularization, by containment in protease- and RNase-resistant vesicles, or by combinations of these and other factors [23,24,26,27,43]. Interestingly, viroids, at about one one-thousandth the size of the smallest known ssRNA virus (see text), are the smallest known self-replicating pathogens of all living species, and both the plant and animal kingdoms have adopted similar ssRNA strategies to store and transmit only the most essential genetic regulatory information in the propagation of either pathological or homeostatic signals. The potential for interaction between various vsRNAs and miRNAs in their hosts, if any, among diverse species of the plant and animal kingdom is currently not known.