Figure 1.
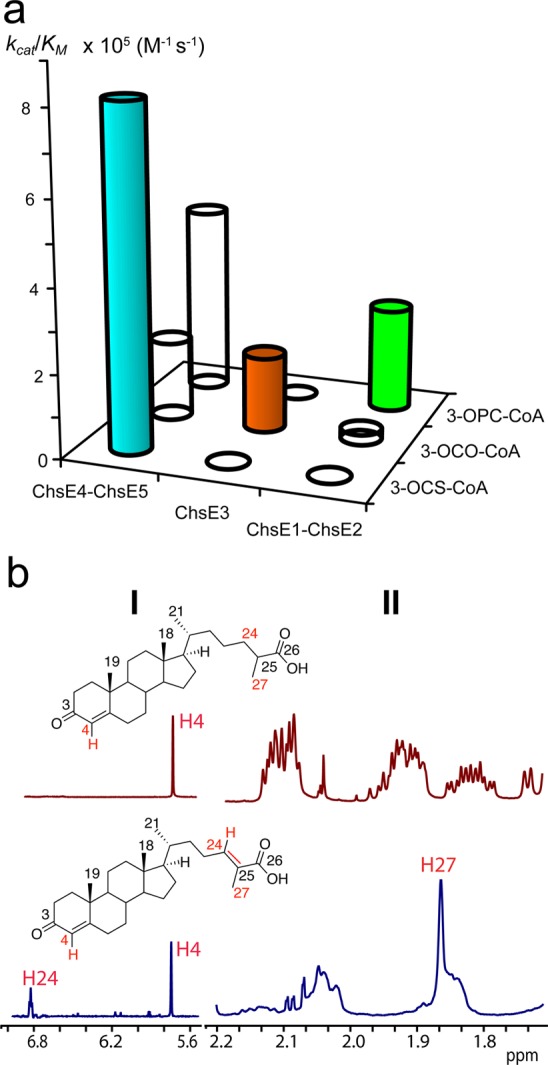
Catalytic specificity for the KstR1-regulated acyl-CoA dehydrogenases and product regio- and stereochemistry for ChsE4-ChsE5. (a) Plot of the catalytic specificity (kcat/KM) of ChsE4-ChsE5, ChsE3, and ChsE1-ChsE2 for the three acyl-CoA metabolic intermediates of cholesterol side chain β-oxidation, 3-OPC-CoA, 3-OCO-CoA, and 3-OCS-CoA, respectively. (b) ChsE4-ChsE5 forms (24E)-3-oxo-cholest-4,24-dien-26-oyl-CoA as determined by the 1H NMR spectra (700 MHz) of substrate precursor 3-oxo-cholest-4-en-26-oic acid and the ChE4-ChsE5 assay product after alkaline hydrolysis of its thioester, top and bottom, respectively. The spectra illustrate the changes in the alkene (I) and methyl (II) regions. The biochemical reaction catalyzed by ChsE4-ChsE5 is shown in Scheme 1.
