Figure 5.
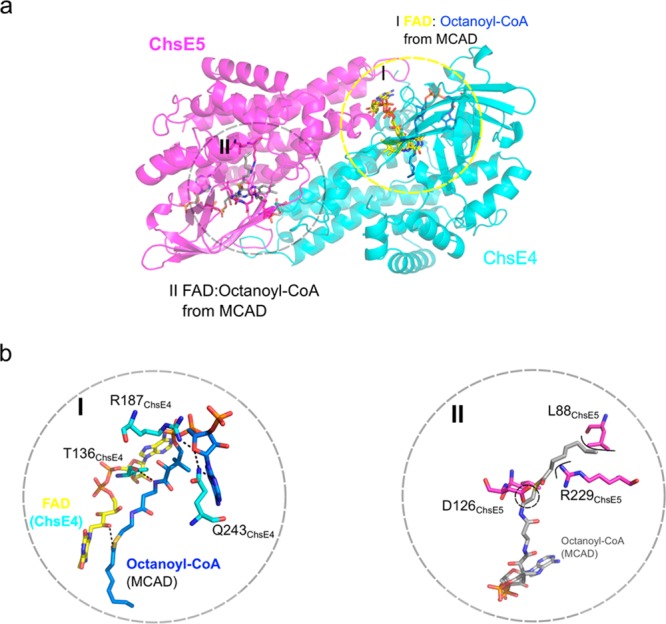
Acyl-CoA binding site. (a) The ChsE4-ChsE5 heterodimer was superimposed onto the MCAD homodimer (PDB: 3MDE) with two FAD/acyl-CoA binding sites. The ChsE4-ChsE5 heterodimer is shown with the two sets of FAD/acyl-CoAs from MCAD. One of the FAD cofactors completely overlays the FAD from ChsE4, circled in I and colored in yellow, and the octanoyl-CoA from MCAD is shown in blue. The other FAD/octanoyl-CoA binding site from MCAD is circled in II and shown in gray. (b) Highly conserved residues T136, R187, and Q243 together with the FAD from ChsE4 interact with the CoA moiety in binding site I. Residues L88, D126, and R229 from ChsE5 would clash with the octanoyl-CoA, shown in circle II. In addition, highly conserved CoA interaction residues T, R, and Q are not conserved in binding site II.
