Figure 6.
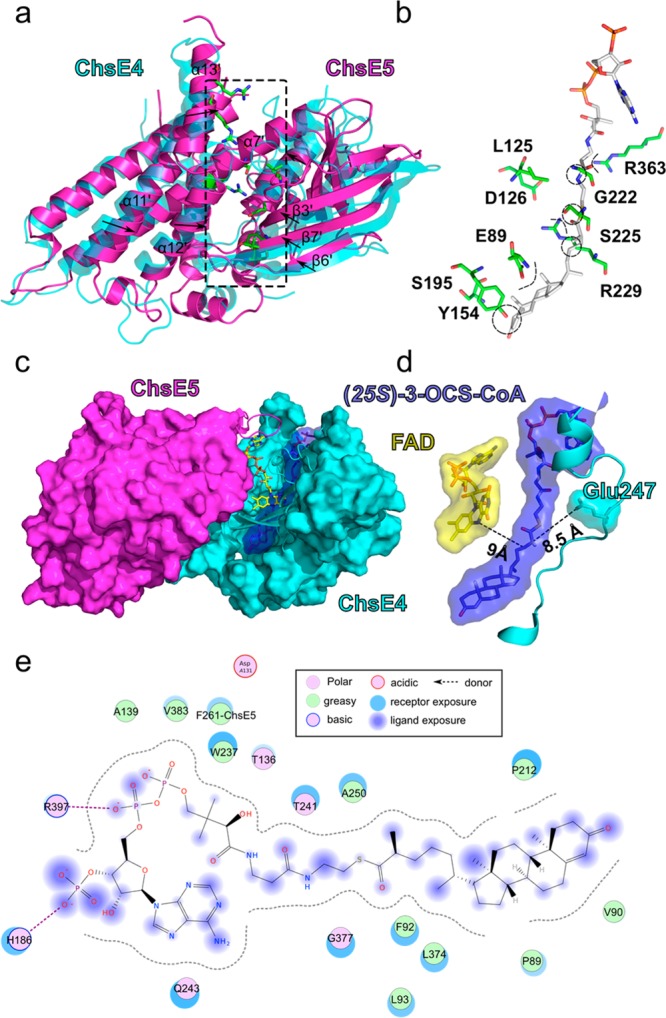
Comparison of ChsE4 and ChsE5 for steroid acyl-CoA binding; model of OCS-CoA bound to the ChsE4-ChsE5 heterodimer. (a) ChsE5 was superimposed onto ChsE4, and the RMSD value is 2.958 Å with 887 α-carbons aligned. The ChsE5 secondary structure that corresponds to the binding tunnel in ChsE4 is shifted into the tunnel relative to ChsE4; the shifting directions are shown by black arrows. The residues that surround the binding tunnel in ChsE5 are shown as sticks and are colored by atom type. (b) Enlarged region of the ChsE5 residues that align with the ChsE4 binding tunnel. These residues have polar, basic, or acidic side chains and clash with the docked substrate (colored by atom) steroid and pantotheine chain. (c) (25S)-OCS-CoA docked into ChsE4 and sandwiched between FAD and the active-site base. For clarity, half of the FAD binding tunnel is represented as a surface colored by a chain, and the other half of the tunnel is drawn as a cartoon. (d) Orientation and proximity of FAD (yellow), the docked 3-OCS-CoA (blue), and the active-site base (light blue). The distances between Glu247 and C25 in 3-OCS-CoA and between C24 in 3-OCS-CoA and N5 in FAD are indicated as dashed lines and labeled. (e) Scheme of the interactions between the ChsE4-ChsE5 heterodimer and (25S)-3-OCS-COA.
