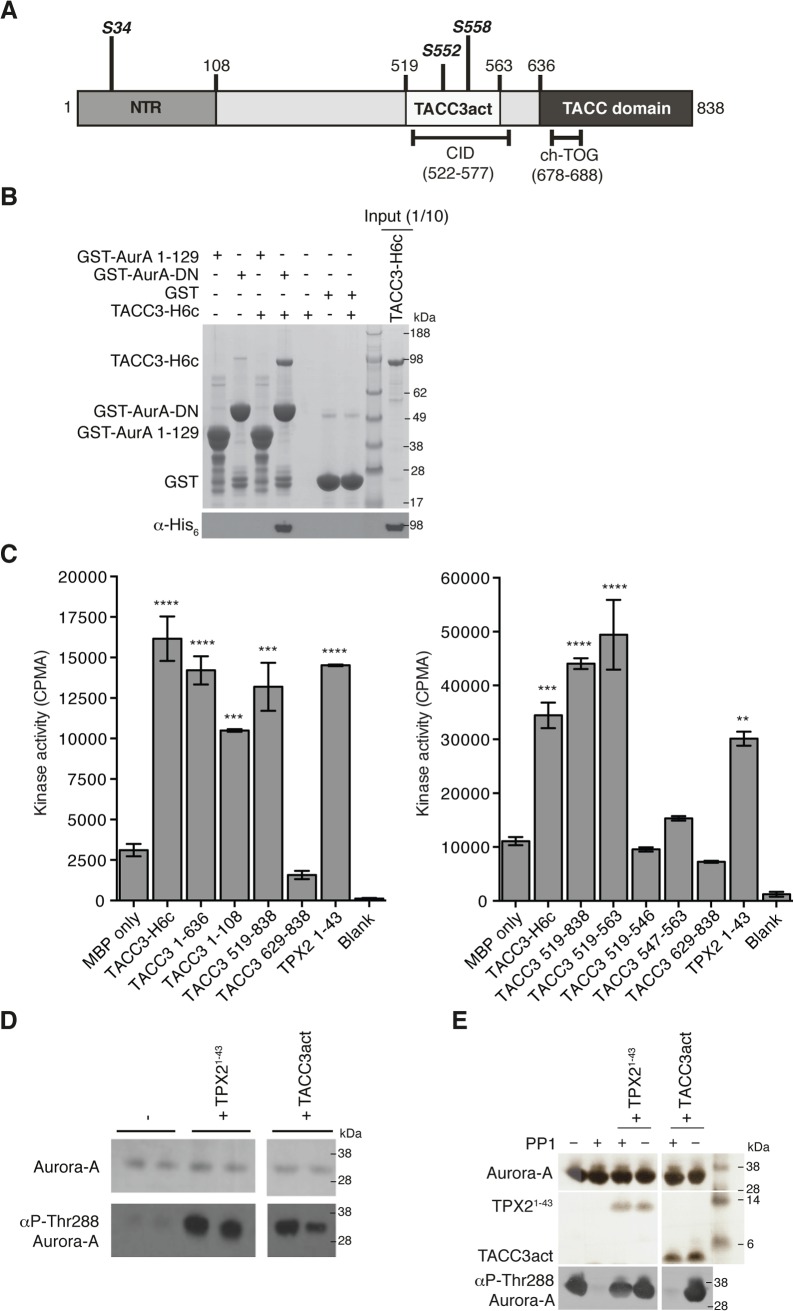
Fig 1. Biochemical characterisation of Aurora-A activation by TACC3.
(A) The domain structure of human TACC3 is shown with conserved regions marked: N-terminal region (NTR, residues 1–108, coloured medium grey), Clathrin Interaction Domain (CID, residues 522–577, marked below), TACC domain (residues 636–838, coloured dark grey). Aurora-A phosphorylation sites are marked in bold italics. Known protein binding regions are marked below. (B) Co-precipitation assay between GST-AurA 1–129, GST-AurA-DN and TACC3-H6c. GST was used as a binding control. Reactions were analysed by SDS-PAGE (top panel). Binding of TACC3-H6c was confirmed by Western blot using an α-His6 antibody (bottom panel). (C) In vitro kinase activity assay of Aurora-A 122–403 in the presence of TACC3-H6c and TACC3 fragments. The known Aurora-A activator, TPX21-43 was used as a positive control. Incorporation of 32P radioisotope into MBP was quantified by scintillation counting. Error bars represent the standard error for two independent reactions. ** = P<0.01, *** = P<0.001 and **** = P<0.0001 using one-way ANOVA with Dunnett's post-hoc test compared to the MBP only reaction. SDS-PAGE analysis of TACC3 proteins used in this assay is shown in S1A Fig. (D) Stimulation of Aurora-A 122–403 autophosphorylation by TPX21-43 and TACC3act. Total Aurora-A is shown in the SDS-PAGE gel (top panel). Levels of phosphorylation were observed by Western blot using an antibody specific to Aurora-A phosphorylated on Thr288 (bottom panel). (E) Protection of Aurora-A 122–403 from dephosphorylation by PP1 in the presence of TPX21-43 and TACC3act. Aurora-A, TPX21-43 and TACC3act were resolved by SDS-PAGE (top panel). Aurora-A phosphorylation was detected by Western blot using a α-phosphoThr288 Aurora-A antibody (bottom panel).