Figure 2.
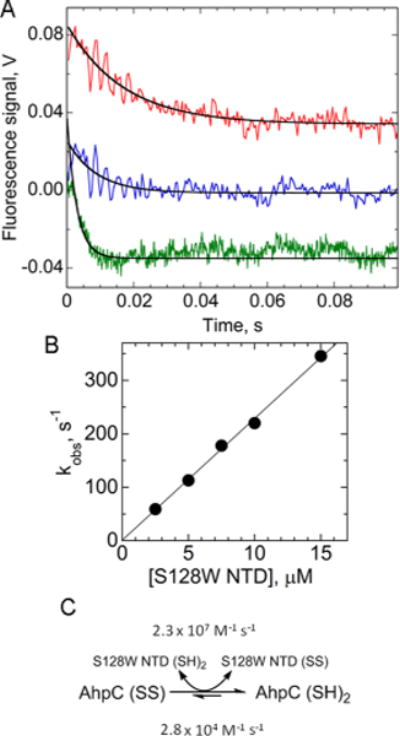
Reduction of AhpC by the redox-sensing mutant of the AhpF N-terminal domain (S128W NTD). (A) To assess rates of reduction of AhpC, oxidized AhpC (1 μM for 2.5–10 μM S128W NTD and 3 μM for >10 μM S128W NTD) was mixed with reduced S128W NTD at 2.5 (red), 5 (blue), and 15 μM (green), monitoring fluorescence changes in both proteins over time (λex = 280 nm; λem > 320 nm). Red and blue curves have been displaced upward on the y-axis for the sake of clarity; amplitudes of change were somewhat variable in these averaged, noisy spectra, but the data were readily fit to a single-exponential equation. (B) Pseudo-first-order rate constants obtained from the fits in panel A, plotted vs S128W NTD concentration, yield a second-order rate constant of 2.3 × 107 M−1 s−1. (C) Reaction pathway annotated with rate constants.
