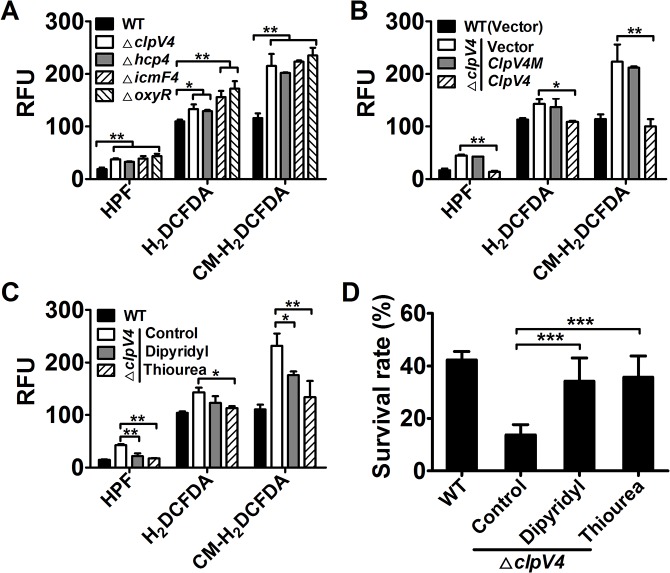
Fig 3. Deletion of T6SS-4 led to accumulation of intracellular ROS in Yptb under oxidative conditions.
A. Oxidative stress induced the generation of intracellular ROS in T6SS-4 mutants. Intracellular ROS in mid-exponential phase bacteria exposed to H2O2 were stained with HPF, CM-H2DCFDA, or H2DCFDA dye; fluorescence signals were measured using a SpectraMax M2 Plate Reader (Molecular Devices) with excitation/emission wavelengths of 490/515 nm (HPF), 495/520 nm (CM-H2DCFDA and H2DCFDA). B. A functional T6SS-4 is required to eliminate cellular ROS. Note the inability of the clpV4M defective in ATPase activity to complement the mutation. C. Reduction of cellular ROS in the mutants by 2,2′-dipyridyl or thiourea. The compound was added to the bacterial cells subjected to oxidative stress challenge and the levels of ROS were measured. D. ROS mitigation agents rescued the sensitivity of T6SS-4 mutants to H2O2. 1 mM 2,2′-dipyridyl or 150 mM thiourea was added to bacterial cells challenged by oxidative stress and their survival rates were determined. In each case, higher levels of cellular ROS were indicated by higher fluorescence intensity. Data shown were the average of three independent experiments; error bars indicate SD from three independent experiments. ***, p<0.001; **, p<0.01; *, p<0.05.