Figure 1.
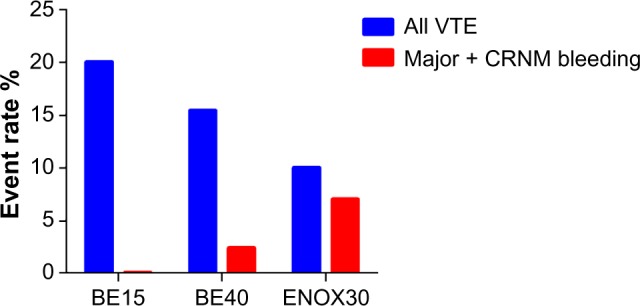
Clinical efficacy and safety of betrixaban in Phase II EXPERT trial.
Notes: The EXPERT trial was a Phase II study comparing oral betrixaban 15 mg bid (BE15), and 40 mg bid (BE40) with enoxaparin 30 mg bid sc (ENOX30) in patients undergoing total knee replacement. All VTE (blue bars) occurred in 20% (95% CI: 11.4%–31.3%), 15% (95% CI: 7.6%–26.5%), and 10% (95% CI: 2.8%–23.7%) of patients receiving betrixaban 15 mg bid, 40 mg bid, and enoxaparin 30 mg bid, respectively. The corresponding clinically relevant bleeding rates (red bars) were 0% (95% CI: 0%–4.2%), 2.4% (95% CI: 0.3%–8.3%), and 7.0% (95% CI: 1.5%–19.1%), respectively.
Abbreviations: VTE, venous thromboembolism; CRNM, clinically relevant non-major; CI, confidence interval.
