Figure 2.
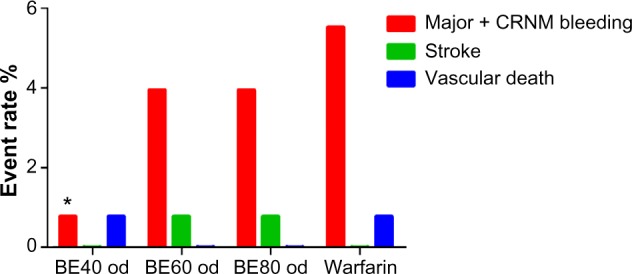
Clinical efficacy and safety of betrixaban in Phase II EXPLORE-Xa trial.
Notes: The EXPLORE-Xa trial was a Phase II study comparing oral betrixaban 40 mg od (BE40), 60 mg od (BE60), and 80 mg od (BE80) with warfarin (adjusted to INR 2.0–3.0) in atrial fibrillation. Clinically relevant bleeding (major bleeding and clinically relevant nonmajor bleeding, red bars) occurred in 0.78% (95% CI: 0.01%–4.76%), 3.94% (95% CI: 1.45%–9.12%), 3.94% (95% CI: 1.45%–9.12%), and 5.51% (95% CI: 2.50%–11.14%) in BE40, BE60, BE80, and warfarin arms, respectively. Only betrixaban 40 mg daily showed statistically significant lower rate of bleeding than warfarin (HR =0.14, *P=0.04). Two ischemic strokes (one each in BE60 and BE80 arms, green bars) and two vascular deaths (one each in BE and warfarin arms, blue bars) occurred.
Abbreviations: CRNM, clinically relevant nonmajor; INR, international normalized ratio; CI, confidence interval; HR, hazard ratio.
