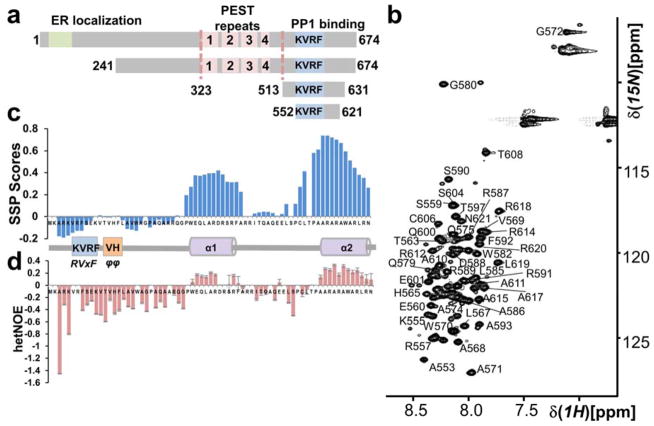
Figure 1. The GADD34 PP1-binding domain is intrinsically disordered.
(a) GADD34 domain structure; ER localization domain, PEST domain (4 PEST repeats; labeled 1 – 4; each ~40 aa) and a PP1-binding domain (includes canonical PP1-binding motif RVxF [KVRF]). GADD34241-674, GADD34513-631 and GADD34552-621 are constructs used. (b) Annotated 2D [1H,15N] HSQC of GADD34552-621. The narrow 1HN chemical shift dispersion is a hallmark of IDPs. (c) Secondary Structure Propensity (SSP) analysis of GADD34552-621 reveals two preferred α-helical secondary structures (helices α1 and α2). (d) Helices α1 and α2 have also reduced fast timescale motions.