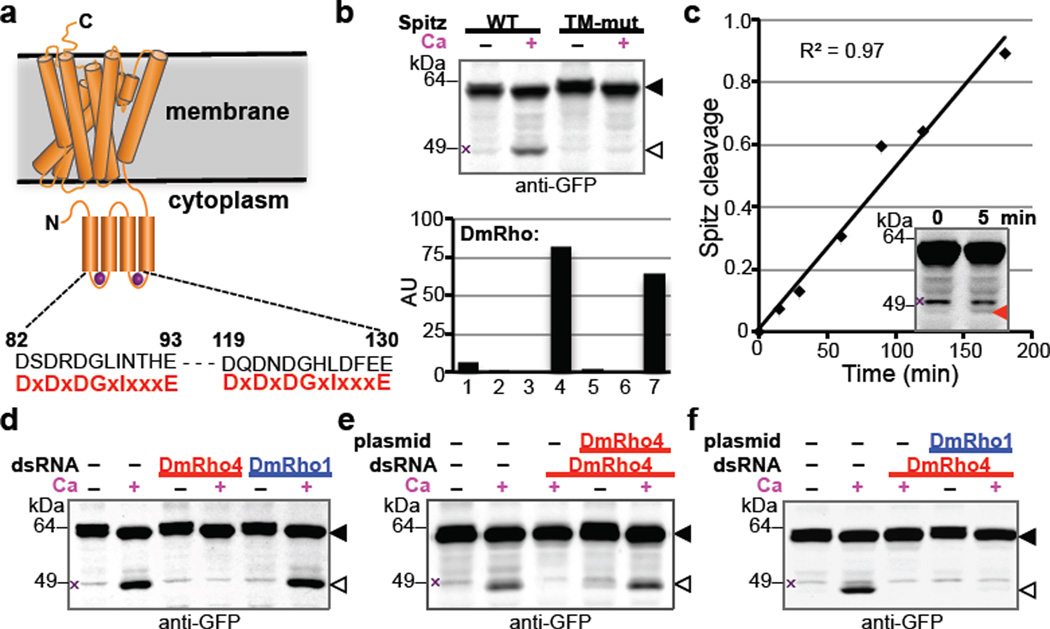
Figure 1. Calcium rapidly stimulates intramembrane proteolysis in Drosophila cells by endogenous DmRho4.
a Diagram comparing the predicted calcium-binding loop residues of DmRho4 to an EF-hand consensus (in red). b Calcium ionophore treatment of Drosophila S2R+ cells induced cleavage of GFP-Spitz, but not its cleavage-site mutant, by endogenous DmRho4. Graph shows expression levels of Drosophila rhomboid genes in S2R+ cells (RNAseq data from modENCODE, modencode.org). c Ionophore-induced Spitz cleavage was detectable within 5 min (red triangle) and linear for 3h. d RNAi knockdown of DmRho4 but not of DmRho1 abrogated calcium-induced cleavage of GFP-Spitz. e Plasmid expression of DmRho4 rescued calcium-induced cleavage of GFP-Spitz in S2R+ cells undergoing RNAi. f Calcium-stimulated Spitz cleavage abolished by DmRho4 RNAi could not be rescued by DmRho1 overexpression. All images are anti-GFP western analyses, with substrate and cleavage bands denoted by black or open triangles, respectively, and non-specific bands marked by × (see Fig. 3d for untransfected cells).