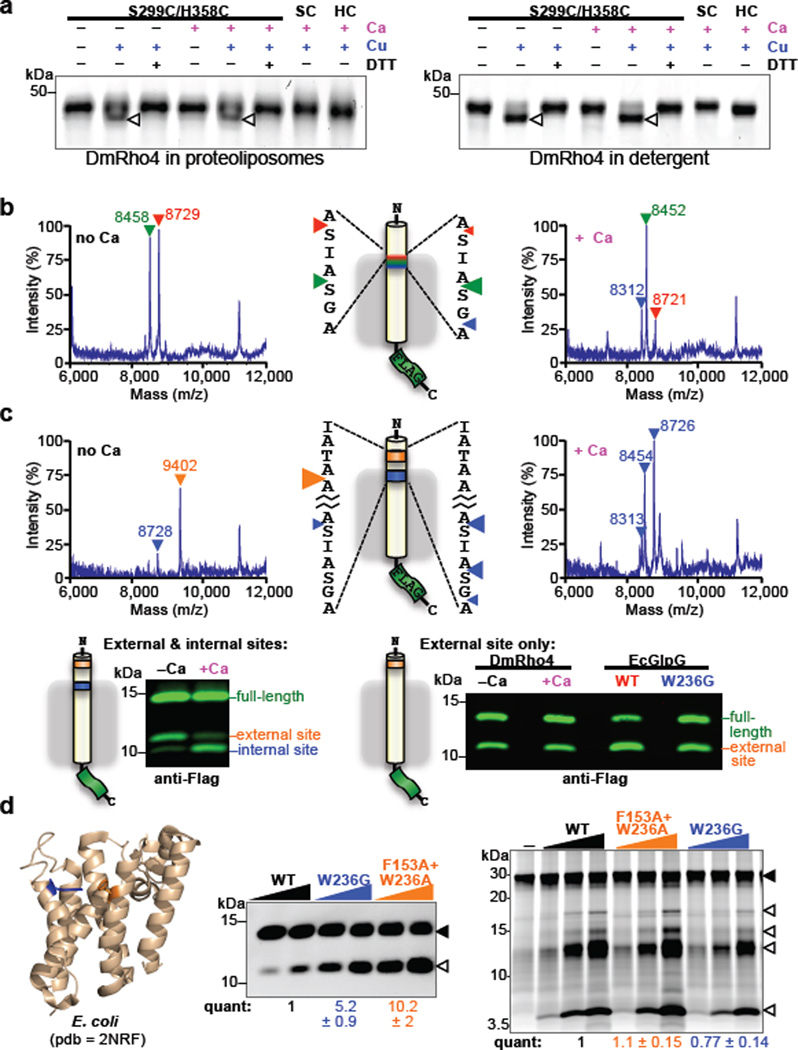
Figure 4. Regulation of intramembrane proteolysis by lateral substrate gating.
a Calcium did not enhance disulfide crosslinking of cysteines installed at the catalytic serine and histidine positions of DmRho4 (triangles indicate crosslinked products). b Shift in substrate cleavage site generated by reconstituted DmRho4 ± calcium as analyzed by mass spectrometry following anti-Flag immunoaffinity capture. Masses and triangles indicating cleavage sites are color-matched. c Processing of a substrate carrying intramembrane (blue) and external (orange) cleavage sites in APP-Flag that was co-reconstituted into liposomes with DmRho4, and assayed ± calcium. Reactions were analyzed by mass spectrometry (top) and quantitative western blotting (lower panels). Processing of a substrate carrying only the external cleavage site was compared for DmRho4 ± calcium, and for EcGlpG versus its gate-open mutant W236G. d Quantitative cleavage analysis of wild type and gate-open mutants of EcGlpG for the APP-Spi7-Flag transmembrane substrate and a soluble BODIPY-casein substrate. Full-length substrate (solid triangles) and cleavage products (open triangles) are indicated.