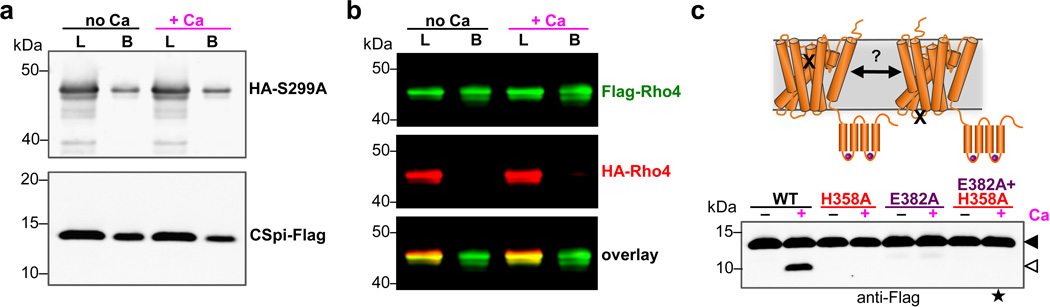
Extended Data Figure 3. Calcium does not regulate DmRho4 through intermolecular interactions.
a Anti-Flag coimmunoprecipitation analysis of HA-DmRho4 and APP-Spi7-Flag substrate from proteoliposomes in the presence or absence of 0.5 mM calcium. An inactive mutant of DmRho4 (S299A) was used to facilitate substrate complex isolation. The amount of HA-tagged DmRo4 co-immunoprecipitated with the Flag-tagged substrate was not affected by the presence of 0.5mM calcium. L denotes ‘load’, B denotes ‘bound’. b Anti-Flag coimmunoprecipitation of Flag-DmRho4 and HA-DmRho4 from proteoliposomes. HA-tagged DmRho4 failed to coimmunoprecipitate with Flag-tagged DmRho4 in both the absence and presence of 0.5 mM calcium. c Mixing a catalytic mutant (S299A) and a calcium-binding mutant (E382A) cannot rescue calcium stimulation in trans (star indicates lane where a product would be expected with the mixed single mutants).