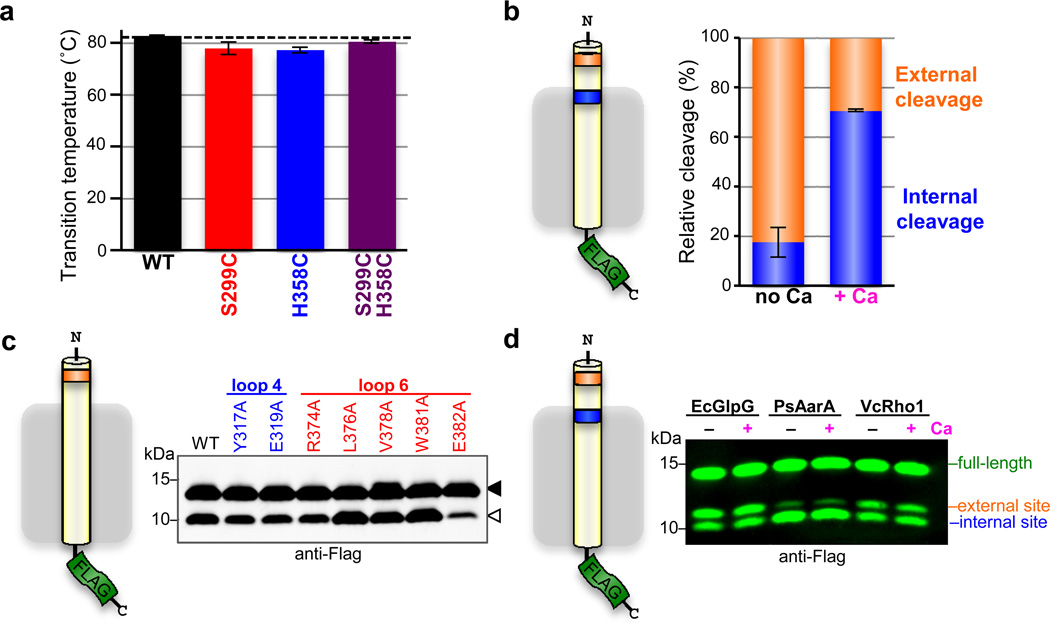
Extended Data Figure 4. Lateral substrate gating underlies direct regulation of intramembrane proteolysis.
a Thermostability analysis of single and double cysteine mutants of DmRho4 (error bars indicate the standard deviation of four experimental replicates). b Average relative proportions of cleavage at the external cleavage site (orange) compared to the internal cleavage site (blue) are shown for DmRho4 in the absence (no Ca) and presence (+ Ca) of 1 mM calcium (error bars indicate standard error of replicate experiments). The external site was favoured in the absence of calcium (approximately 80%) while internal cleavage was preferred in the presence of calcium (approximately 70%). c DmRho4 loop 4 and loop 6 calcium-binding site mutants retained calcium-independent cleavage of a substrate harbouring only an external cleavage site. Full-length substrate (solid triangle) and cleavage product (open triangle) are indicated. c Cleavage of a substrate with external and internal cleavage sites was compared for E. coli GlpG, P.stuartii AarA, and V. cholerae Rho1 in the absence (no Ca) or presence (+ Ca) of 0.5 mM calcium. The relative proportions of cleavage at the two sites varied between the bacterial rhomboid proteases, but in no case did calcium alter the cleavage site preference.