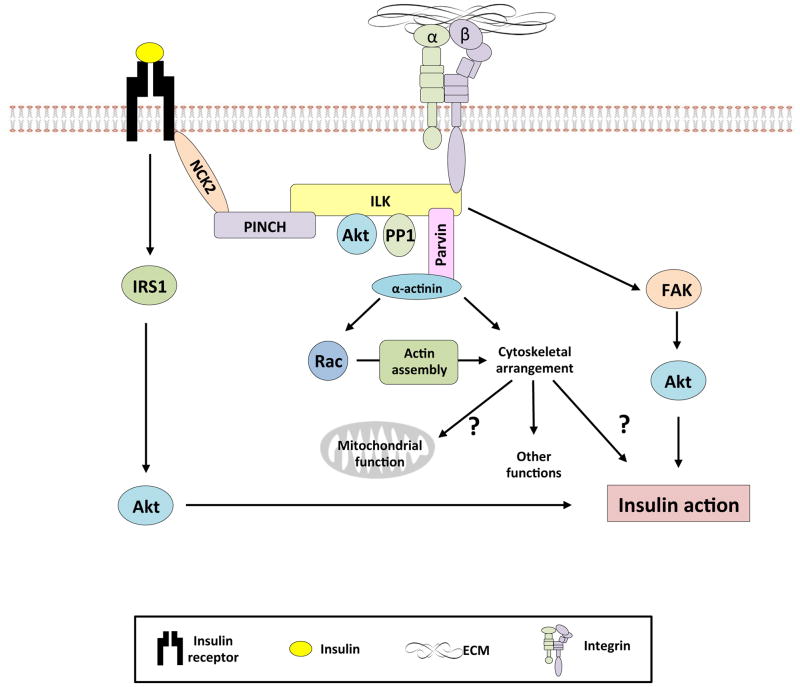
Figure 2.
The role of integrin α2β1 diet-induced muscle insulin resistance.
In the HF-fed state, capillary density and endothelial function are impaired. This results in decreased potential for glucose and insulin transport into the interstitial space despite hyperglycemia and hyperinsulinemia. Moreover, increased ECM deposition in the interstitial space also provides a physical barrier to glucose and insulin transport to the myocyte. Insulin signaling within the myocyte is impaired and this may be attributed to increased integrin α2β1 signaling as a consequence of increased deposition of the ECM. This results in impaired Glut4 translocation and decreased glucose transport into the myocyte. In contrast, the genetic deletion of the integrin α2 subunit results in improved insulin-stimulated muscle glucose uptake.