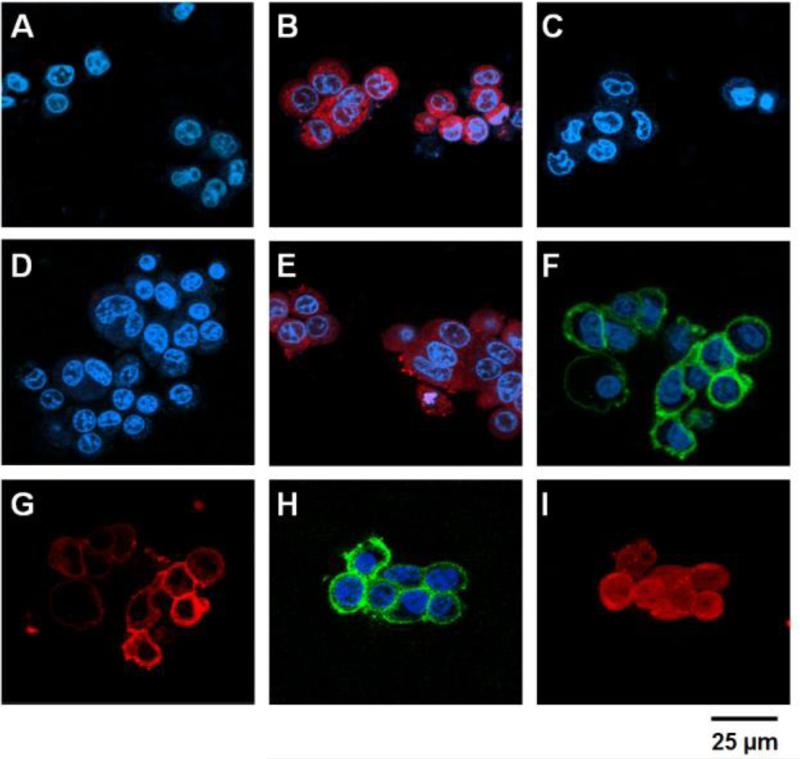
Figure 3.
Confocal microscopic analyses of the PTD-modified ATTEMPTS. HCT116 (A - C) and LS174T cells (D – I) were incubated with either: 1) TRITC-labeled gelonin (A & D), 2) TRITC-labeled TAT-Gel (B & E), 3) TRITC-labeled TAT-Gel/Dylight 488-labeled T84.66-Hep (a.k.a. TAT-Gel/T84.66-Hep) complex (C, F & G) or 4) the TAT-Gel/T84.66-Hep complex with protamine (H & I), and, after counterstaining the nuclei with Hoechst 33342, the fluorescence images of the cells were acquired (Hoechst: blue, TRITC: red and Dylight 488: green). Whereas gelonin entered neither HCT116 (A) nor LS174T (D) cells, a significant uptake of TAT-Gel was observed in both the cell lines (B & E). In a sharp contrast, when the cells were treated with TAT-Gel/T84.66-Hep complex, TAT-Gel was unable to internalize. While TAT-Gel/T84.66-Hep did not bind to HCT116 cells (C), TAT-Gel/T84.66-Hep bound to the LS174T cells (T84.66-Hep: F; TAT-Gel: G), but little internalization. However, to the TAT-Gel/T84.66-Hep-treated LS174T cells, after addition of protamine, TAT-Gel was able to internalize the cells (I), with T84.66-Hep remaining on the cell surface (H). (TAT-Gel: recombinant TAT-gelonin fusion chimera, T84.66-Hep: T84.66-heparin chemical conjugate)