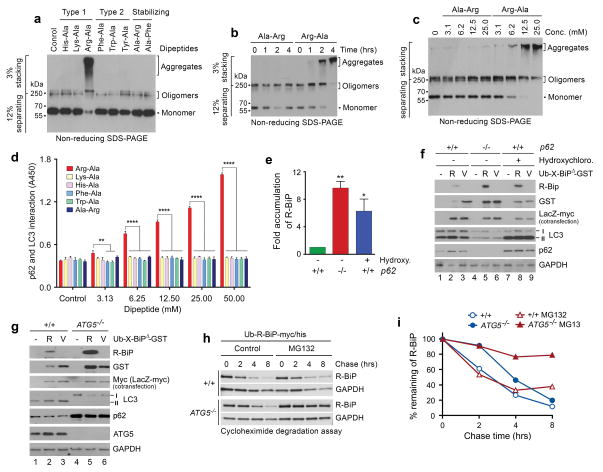
Figure 6.
The Nt-Arg residue induces oligomerization and aggregation of p62 in vitro. (a) In vitro oligomerization/aggregation assays of HEK293 cell lysates expressing p62-myc/his. The dipeptide Arg-Ala (type-1) at a final concentration of 20 mM was added to the extracts (1 μg) in comparison with other dipeptides. (b, c) Similar to a. Arg-Ala was compared with Ala-Arg in a time-dependent (b) and dose-dependent (c) manner. (d) The p62-LC3 interaction assay shows that Nt-Arg of Arg-Ala promotes the interaction of p62 with LC3-GST. LC3-GST (3 μg) was immobilized to gluthathione (GSH)-coated wells and incubated with HEK293 cell extracts (20 μg) expressing p62. Following incubation with dipeptides for 2 hrs, bound p62 was detected using anti-p62 antibody. Quantification is of n = 3 independent experiments; bars represent mean ± s.d. Statistical significance was calculated using a one-way ANOVA test (N.S. P > 0.05; ** P < 0.01; **** P < 0.0001. (e) Quantitation of f. Quantification is of n = 3 independent experiments; bars represent mean ± SEM. Statistical significance was calculated using a one-way ANOVA test (* P < 0.05; ** P < 0.01). (f) R-BiPΔ-GST, but not V-BiPΔ-GST, is metabolically stabilized by p62 knockout or pharmaceutical inhibition of autophagy. Shown is immunoblotting of X-BiPΔ-GST (X= Arg or Val) in +/+ and p62−/− MEFs with or without the treatment of 25 μM hydroxychloroquine for 16 hrs. Note that the level of R-BiPΔ-GST (as determined by GST immunoblotting) is lower as compared with V-BiPΔ-GST because of its degradation (lanes 2 vs. 3) and increased in p62-deficient cells (lanes 2 vs. 5) or by the treatment of hydroxychloroquine. (g) R-BiPΔ-GST, but not V-BiPΔ-GST, is metabolically stabilized in ATG5−/− MEFs. Shown is immunoblotting of X-BiP19–124-GST (X-BiPΔ-GST, X= Arg or Val) in +/+ and ATG5−/− MEFs. Note that the level of R-BiPΔ-GST, as determined by GST immunoblotting, is lower than V-BiP19–124-GST (lanes 2 vs. 3) and increased in ATG5-deficient cells (lanes 2 vs. 5). (h) Cycloheximide degradation assay of R-BiP-myc/his generated from Ub-R-BiP-myc/his in +/+ and ATG5−/− MEFs in the absence or presence of 5 μM MG132. Cells were treated with 50 μg/ml cycloheximide, followed by time-course immunoblotting of R-BiP. (i) Quantitation of h. Shown is a representative of two independent experiments.