Figure 5.
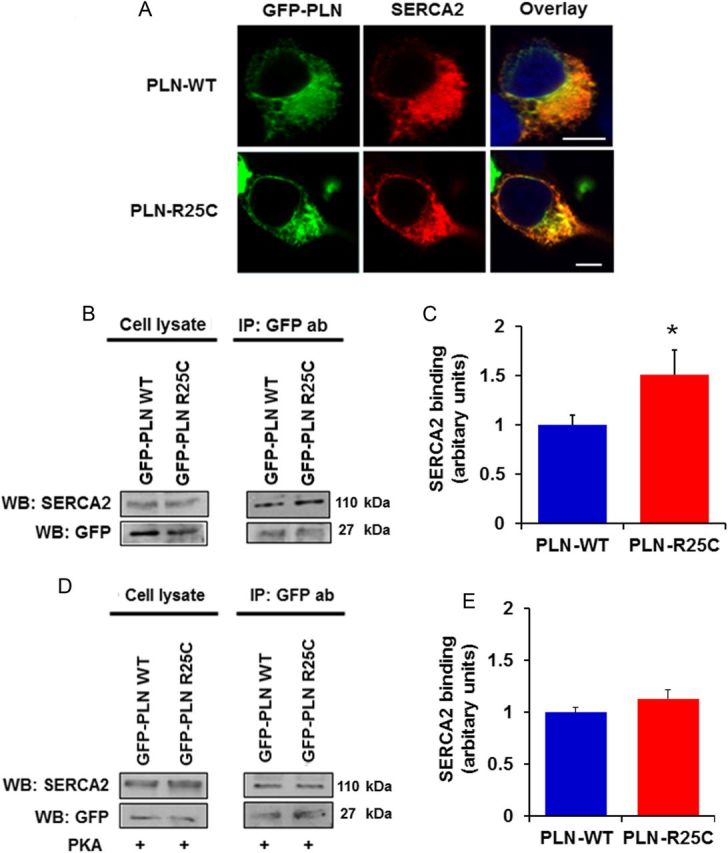
(A) The R25C-PLN mutant co-localizes with SERCA2 in transfected HEK 293 cells, similar to WT-PLN. Nuclei are stained with DAPI. Scale bar, 5 μm; (B and C) R25C-PLN mutant exhibits enhanced association to SERCA2. Immunoprecipitation assays in HEK 293 cells that co-express GFP-PLN and SERCA2 were performed using GFP antibody. Quantification of SERCA2 levels revealed a significant increase in the SERCA2/R25C-PLN protein complex compared with GFP-WT-PLN (n = 4) values are means ± SE; *P < 0.05, compared with WT-PLN); (D and E) enhanced binding of R25C-PLN with SERCA2a was abolished upon PKA phosphorylation. Immunoprecipitations were performed in lysates from HEK 293 transfected cells that had been previously phosphorylated with PKA. Western blot analysis (D) determined similar levels of SERCA2 in both WT-PLN and R25C-PLN samples and quantitative analysis (E) showed no difference in SERCA2 binding between WT-PLN and R25C-PLN (n = 4).
