Abstract
Real-time quantitative PCR (RT-qPCR) is a reliable and widely used method for gene expression analysis. The accuracy of the determination of a target gene expression level by RT-qPCR demands the use of appropriate reference genes to normalize the mRNA levels among different samples. However, suitable reference genes for RT-qPCR have not been identified in Sacha inchi (Plukenetia volubilis), a promising oilseed crop known for its polyunsaturated fatty acid (PUFA)-rich seeds. In this study, using RT-qPCR, twelve candidate reference genes were examined in seedlings and adult plants, during flower and seed development and for the entire growth cycle of Sacha inchi. Four statistical algorithms (delta cycle threshold (ΔCt), BestKeeper, geNorm, and NormFinder) were used to assess the expression stabilities of the candidate genes. The results showed that ubiquitin-conjugating enzyme (UCE), actin (ACT) and phospholipase A22 (PLA) were the most stable genes in Sacha inchi seedlings. For roots, stems, leaves, flowers, and seeds from adult plants, 30S ribosomal protein S13 (RPS13), cyclophilin (CYC) and elongation factor-1alpha (EF1α) were recommended as reference genes for RT-qPCR. During the development of reproductive organs, PLA, ACT and UCE were the optimal reference genes for flower development, whereas UCE, RPS13 and RNA polymerase II subunit (RPII) were optimal for seed development. Considering the entire growth cycle of Sacha inchi, UCE, ACT and EF1α were sufficient for the purpose of normalization. Our results provide useful guidelines for the selection of reliable reference genes for the normalization of RT-qPCR data for seedlings and adult plants, for reproductive organs, and for the entire growth cycle of Sacha inchi.
Keywords: Plukenetia volubilis, reference gene, RT-qPCR, flower development, seed development, biofuels
1. Introduction
Sacha inchi (Plukenetia volubilis L.), a member of the Euphorbiaceae, is native to the rain forest of South America [1,2]. Because its seed oil is rich in polyunsaturated fatty acids (PUFAs) and lipovitamins, Sacha inchi has great potential economic value to the food and pharmaceutical industries [3,4]. Moreover, Sacha inchi oil is also a good feedstock for biodiesel production [5]. To promote gene function studies in Sacha inchi, transcriptomic analysis has been performed for the period of seed development, and numerous key genes involved in the regulation of seed oil biosynthesis have been identified [6]. A good characterization of expression profiles of these key genes will facilitate a better understanding of gene function in seed oil biosynthesis.
Characterized by high sensitivity, specificity and accuracy, real-time quantitative PCR (RT-qPCR) has become the preferred method for detecting and measuring gene expression [7,8,9]. A prerequisite for the reliable analysis of gene expression is the normalization of RT-qPCR data, which can minimize the non-specific variations caused by variations in the quantity and quality of mRNA and variations in the efficiencies of reverse transcription and PCR [10,11,12,13]. Therefore, the selection of appropriate reference genes as internal controls that are expressed at constant levels among tissues and over time is very important.
In the last decade, several statistical algorithms have been developed for the selection of reference genes for RT-qPCR analysis, such as the delta cycle threshold (ΔCt) [14], geNorm [15], BestKeeper [16] or NormFinder [17] algorithms. The ΔCt method ranks the candidate genes by comparing the relative expression of pairwise under a given set of experimental conditions [14]. The ΔCt method indicated the mean of standard deviation (SD) of each candidate reference genes, and the candidate with the lowest SD value was proposed to be the most stable gene [14]. The geNorm is a Visual Basic application tool that relies on the principle that the expression ratio of two perfect reference genes should be constant under different development stages or in various plant tissues. The expression stability (M) is calculated based on the average pairwise variation between all reference genes tested. The gene with a lower M value indicated the gene expression is more stable [15]. The BestKeeper program evaluates the most stably expressed genes based on the coefficient of variance (CV) and SD of the quantification cycle (Cq) values. The lower coefficient of variance and standard deviation (CV ± SD) indicated the gene expression was more stable [16]. The NormFinder program is based on a variance estimation approach, which ranks the candidate genes according to the stability of a gene under a given set of experimental conditions compared to the rest of the tested genes. The more stably expressed genes are indicated by the lower average expression stability values (M values) [17]. The application of these algorithms has simplified the identification of reliable reference genes by enabling the rapid calculation of the expression stability and the determination of the optimal number of reference genes required for normalization [18,19].
The identification of optimal reference genes for RT-qPCR has been reported for several plants, including bamboo [20], Jatropha curcas [21], coffee [22], oil palm [23], peach [24] and Petunia hybrida [25]. However, a number of commonly used housekeeping genes, such as actin (ACT), elongation factor 1alpha (EF1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and ubiquitin(UBQ), is insufficient for RT-qPCR normalization because of variations in expression in different species, tissues, developmental stages or environmental conditions. For sesame, SiACT was recommended as the reference gene for seed development and germination, although SiUBQ6 was better for bud development [26]. For Chinese cabbage, EF1α was reported to be the best reference gene among five tissues, and GAPDH was most suitable for drought stress conditions [27]. The 18S rRNA (18S), ACT and GAPDH genes were reported to be expressed unstably in papaya (Carica papaya) under numerous experimental conditions [28]. Hence, the selection of multiple housekeeping reference genes is required for the accurate normalization of gene expression levels under varied experimental conditions.
For this study, in order to reduce the likelihood that the reference genes exhibited regulated co-variation, a group of genes with varied roles in different cellular processes were chosen (Table 1). The expression stabilities of twelve candidate reference genes (18S, ACT, CYC, EF1α, GAPDH, PLA, RPII, RPS13, TEF2, TUB, UBL and UCE) were examined in Sacha inchi seedlings and adult plants, during flower and seed development, and for the entire growth cycle of Sacha inchi. Our results indicate that traditional housekeeping genes were less stably expressed than other reference genes in the given experimental datasets.
Table 1.
Selected candidate reference genes, primer sequences and PCR amplification characteristics.
| Gene/GenBank Accession Number | Description | Function | Forward (F) and Reverse (R) Primer Sequences (5′→3′) | Amplicon Length | Tm (°C) | Amplification Efficiency (%) | Correlation Coefficient |
|---|---|---|---|---|---|---|---|
| 18S/KP729648 | 18S ribosomal RNA | ribosomal structure | F: ACCAGGTCCAGACATAGTAAGGATTGA | 140 bp | 81.73 | 106.40 | 0.999 |
| R: AGTTAGCAGGCTGAGGTCTCGTT | |||||||
| ACT/GADC01011038 | actin | cytoskeletal structural protein | F: CCAGAAGTCTTGTTCCAGCCATCTC | 185 bp | 80.66 | 105.78 | 0.999 |
| R: GCGGTGATCTCCTTGCTCATACG | |||||||
| CYC/GADC01018836 | cyclophilin | protein folding | F: GGCAAGATACGAACGGATCACAGTT | 145 bp | 82.95 | 108.93 | 0.999 |
| R: GGCACTCCACTCCGACTTCCTT | |||||||
| EF1α/GADC01006492 | elongation factor 1-alpha | protein biosynthesis | F: GGTATTCTCAAGCCTGGTATGGTTGT | 102 bp | 80.48 | 94.98 | 0.999 |
| R: GAGAGCCTCCTGAAGAGCCTCAT | |||||||
| GAPDH/GADC01052274 | glyceraldehyde-3-phosphate dehydrogenase | glucose metabolism | F: TGGCAAGCATATTCAGGCAGGAG | 116 bp | 81.63 | 94.98 | 0.999 |
| R: TTGGCTCATCAGGATTGTAGGTATCAG | |||||||
| PLA/KP729647 | phospholipase A22 | lipid catabolic process | F: ATACCATACAGAACGCAGCTTGTGAA | 101 bp | 79.92 | 103.33 | 0.998 |
| R: TTCCGCCAGTTCCAACCTATCCA | |||||||
| RPII/GADC01020629 | RNA polymerase II subunit | mRNA process | F: GCCTCGGTCTCATTCCTCTTACAAG | 109 bp | 82.44 | 104.17 | 0.999 |
| R: AACTCAACAGAACAATACTCGCACTGA | |||||||
| RPS13/GADC01008223 | 30S ribosomal protein S13 | DNA-templated transcription | F: TAATGCACAGCTTCCAGATGAC | 202 bp | 81.47 | 90.55 | 0.999 |
| R: AACCAGTCGCTTTGATTCTTCT | |||||||
| TEF2/GADC01000224 | transcription elongation factors-II | transcription | F: AGATTCAGAGCATGAAGAGGGAC | 182 bp | 82.18 | 104.17 | 0.996 |
| R: CGATCGGTATTTGTTGCGATTT | |||||||
| TUB/GADC01018931 | Tubulin beta-4 chain | structural constituent of cytoskeleton | F: ACAATTCACTGCCATGTTCAGGAGAA | 169 bp | 82.05 | 97.83 | 0.999 |
| R: GTCATCTTCGTAGTCACCTTCGTCATC | |||||||
| UBL/GADC01024109 | ubiquitin-like | protein binding | F: GCTACGTCTGCGTGGAGGAATG | 197 bp | 82.39 | 99.53 | 0.996 |
| R: TGTAGTCTGCCAATGTGCGTCC | |||||||
| UCE/GADC01034781 | ubiquitin-conjugating enzyme | ubiquitin-dependent protein catabolic process | F: TGGAATGGATGACGGAGACGACAT | 142 bp | 78.74 | 100 | 0.997 |
| R: AACACTTGGTGGCTTCTCTGGATAATC |
2. Results
2.1. Specificity and Efficiency of PCR Amplification of the Candidate Reference Genes
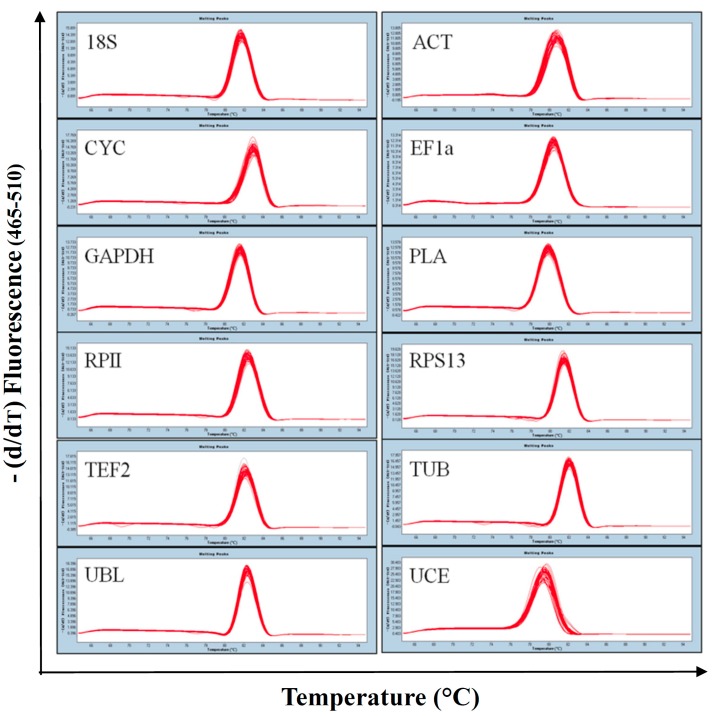
A total of twelve candidate reference genes (18S, ACT, CYC, EF1α, GAPDH, PLA, RPII, RPS13, TEF2, TUB, UBL and UCE) were selected to normalize the gene expression levels in Sacha inchi using RT-qPCR. The specificity of the primers (Table 1, Supplementary Figure S1) was confirmed by the single peak melting curves of the qPCR products (Figure 1) and the presence of a single band at the correct size for each primer pair in 2% agarose gel electrophoresis (Supplementary Figure S2). The melting temperatures of the PCR products all ranged between 78.74 °C for UCE and 82.95 °C for CYC (Table 1). The amplification efficiencies ranged from 90.55% for RPS13 to 108.93% for CYC, and the correlation coefficients (R2) for the primers all ranged between 0.996 and 0.999 (Table 1).
Figure 1.
Figure 1. Melting curves for the twelve candidate reference genes. The melting temperature of each amplicon is visualized by plotting the negative derivative of the change in fluorescence divided by the change in temperature in relation to the temperature (−(d/dT) Fluorescence (465–510)).
2.2. Transcript Accumulation of Candidate Reference Genes
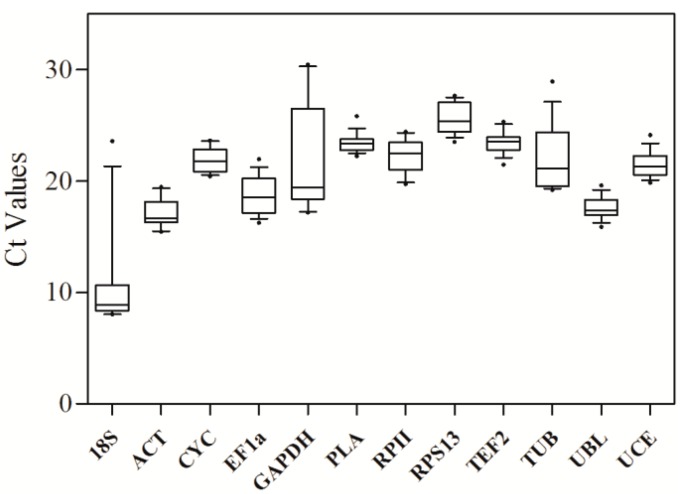
The transcript levels of the twelve candidate reference genes, presented as the cycle threshold (Ct) values, were obtained by RT-qPCR. The box-plot analysis was performed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA, USA). The data used to produce the box-plot were shown in the supplementary Table S1. The results indicated that the candidate reference genes evaluated in this study encompassed a wide range of Ct values, ranging from 8 to 30, with the majority ranging from 16 to 27 (Figure 2, Supplementary Table S1). The 18S gene was the most abundant reference gene in the tested Sacha inchi tissues with the lowest mean Ct value of 9, whereas RPS13 was the least abundant reference gene with the highest mean Ct value of 25. The results also revealed that the PLA gene was characterized by the smallest variation in transcript levels among plant tissues, whereas the GAPDH gene displayed the largest variation among tissues.
Figure 2.
Average cycle threshold (Ct) values for the twelve candidate reference genes. Boxes indicate the interquartile range. Lines across the boxes indicate the average Ct values. Whiskers represent 95% confidence intervals, and black dots represent outliers.
The expression profiles of twelve candidate reference genes in various tissues are displayed in Supplementary Table S1. The 18S gene was stably expressed in most tissues with high levels except in adult young leaves, and much lower levels in seeds at 90 and 130 days after pollination (DAP). RPII had relatively high abundance in young tissues. The expression of EF1α, RPS13 and UCE varied during seed development. Across all the tissues tested, GAPDH and TUB had obvious expression variation, whereas ACT, CYC, PLA, UBL and TEF2 had relatively stable expression.
2.3. Ranking of Candidate Reference Genes and Determination of the Optimal Reference Genes
In this study, to perform an all-sided analysis, the twelve candidate reference genes were evaluated in five experimental sets comprising samples collected at defined developmental stages. The first experimental set consisted of roots, stems, young leaves and mature leaves from three-week-old seedlings. The second set consisted of roots, stems, young leaves, mature leaves, young inflorescences and seeds (90 DAP) from one-year-old adult plants. The developmental stages of the flower (inflorescence buds and young inflorescences, female and male flowers) and seed (15, 40, 90 and 130 DAP) were included in the third and fourth experimental sets, respectively. The entire life cycle of Sacha inchi was analyzed in the fifth experimental set comprising all 16 samples described above. To obtain higher-accuracy stability rankings, four statistical algorithms (ΔCt method, BestKeeper, NormFinder and geNorm) were applied to assess the Ct values for each candidate reference gene. The result indicated that the most appropriate reference genes differed among these statistical algorithms, whereas the identities of the inappropriate reference genes were largely consistent among the tested algorithms (Table 2 and Supplementary Table S2). The RefFinder, a web-based comprehensive tool integrating the above mentioned four computational programs (Available online: http://www.leonxie.com/referencegene.php), was also employed to calculate the recommended comprehensive ranking order. For the first experimental set (three-week-old seedlings), UCE and ACT were the two most stable reference genes based on the ΔCt, NormFinder and geNorm analyses, whereas PLA and 18S were the two best reference genes based on the BestKeeper. According to the calculation performed by RefFinder, UCE, ACT and PLA were the three most stable reference genes in the seedling of Sacha inchi, whereas GAPDH, TUB and UBL were the least stable genes (Table 2).
Table 2.
Stability ranking of candidate reference genes in different developmental stages.
| Analysis Tool | Ranking Order (The 1st is the most stable, and the 12th is the least stable) | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||||||||||||||
| Seedling | |||||||||||||||||||||||||
| ΔCt | UCE | ACT | CYC | PLA | 18S | RPS13 | EF1α | TEF2 | RPII | UBL | TUB | GAPDH | |||||||||||||
| BestKeeper | PLA | 18S | TEF2 | ACT | UCE | CYC | UBL | RPS13 | RPII | EF1α | TUB | GAPDH | |||||||||||||
| NormFinder | UCE | ACT | EF1α | RPS13 | CYC | RPII | PLA | TUB | 18S | TEF2 | UBL | GAPDH | |||||||||||||
| geNorm | ACT | UCE | PLA | 18S | TEF2 | CYC | RPS13 | UBL | EF1α | RPII | TUB | GAPDH | ||||||||||||||
| Recommended comprehensive ranking | UCE | ACT | PLA | 18S | CYC | TEF2 | RPS13 | EF1α | RPII | UBL | TUB | GAPDH | |||||||||||||
| Adult Plant | |||||||||||||||||||||||||
| ΔCt | RPS13 | CYC | EF1α | RPII | UCE | ACT | UBL | TEF2 | PLA | TUB | GAPDH | 18S | |||||||||||||
| BestKeeper | UCE | PLA | UBL | CYC | TEF2 | RPS13 | ACT | RPII | EF1α | TUB | GAPDH | 18S | |||||||||||||
| NormFinder | EF1α | ACT | RPII | RPS13 | CYC | UCE | PLA | UBL | TEF2 | TUB | GAPDH | 18S | |||||||||||||
| geNorm | CYC | RPS13 | EF1α | RPII | UCE | ACT | UBL | TEF2 | PLA | TUB | GAPDH | 18S | ||||||||||||||
| Recommended comprehensive ranking | RPS13 | CYC | EF1α | UCE | RPII | ACT | PLA | UBL | TEF2 | TUB | GAPDH | 18S | |||||||||||||
| Flower Development | |||||||||||||||||||||||||
| ΔCt | PLA | UCE | ACT | GAPDH | TEF2 | EF1α | CYC | RPII | UBL | 18S | RPS13 | TUB | |||||||||||||
| BestKeeper | ACT | PLA | GAPDH | UCE | TEF2 | 18S | CYC | UBL | EF1α | RPII | RPS13 | TUB | |||||||||||||
| NormFinder | UCE | PLA | TEF2 | EF1α | ACT | GAPDH | RPII | CYC | RPS13 | UBL | 18S | TUB | |||||||||||||
| geNorm | ACT | GAPDH | PLA | UCE | TEF2 | CYC | UBL | EF1α | 18S | RPII | RPS13 | TUB | ||||||||||||||
| Recommended comprehensive ranking | PLA | ACT | UCE | GAPDH | TEF2 | EF1α | CYC | UBL | RPII | 18S | RPS13 | TUB | |||||||||||||
| Seed Development | |||||||||||||||||||||||||
| ΔCt | UCE | RPS13 | EF1α | ACT | RPII | CYC | UBL | PLA | TEF2 | GAPDH | TUB | 18S | |||||||||||||
| BestKeeper | UBL | TEF2 | PLA | CYC | ACT | RPII | RPS13 | UCE | EF1α | GAPDH | TUB | 18S | |||||||||||||
| NormFinder | UCE | EF1α | RPS13 | RPII | ACT | CYC | UBL | PLA | GAPDH | TEF2 | TUB | 18S | |||||||||||||
| geNorm | RPII | RPS13 | EF1α | UCE | ACT | CYC | UBL | PLA | TEF2 | GAPDH | TUB | 18S | ||||||||||||||
| Recommended comprehensive ranking | UCE | RPS13 | RPII | EF1α | UBL | ACT | CYC | PLA | TEF2 | GAPDH | TUB | 18S | |||||||||||||
| Entire Growth Cycle | |||||||||||||||||||||||||
| ΔCt | UCE | ACT | EF1α | CYC | RPII | RPS13 | PLA | UBL | TEF2 | TUB | 18S | GAPDH | |||||||||||||
| BestKeeper | PLA | UBL | TEF2 | UCE | CYC | ACT | RPS13 | RPII | EF1α | TUB | 18S | GAPDH | |||||||||||||
| NormFinder | EF1α | ACT | UCE | RPII | CYC | RPS13 | PLA | UBL | TEF2 | TUB | 18S | GAPDH | |||||||||||||
| geNorm | ACT | UCE | CYC | EF1α | RPII | RPS13 | UBL | PLA | TEF2 | TUB | 18S | GAPDH | ||||||||||||||
| Recommended comprehensive ranking | UCE | ACT | EF1α | CYC | PLA | RPII | UBL | RPS13 | TEF2 | TUB | 18S | GAPDH | |||||||||||||
For the adult plant set (Table 2), RPS13 and CYC were the most stable reference genes according to the recommendations of ΔCt and geNorm algorithms, UCE and PLA were recommended by BestKeeper, and EF1α, ACT and RPII were recommended by NormFinder. The comprehensive ranking order indicated that RPS13, CYC and EF1α were the three most stably expressed reference genes. The 18S, GAPDH and TUB genes clearly showed the most variable expression levels.
During flower development, PLA and UCE were identified to be the most stable reference genes by ΔCt and NormFinder, whereas ACT, PLA and GAPDH were identified by BestKeeper and geNorm. The comprehensive ranking order suggests that the PLA, ACT and UCE genes were the optimal reference genes and that the 18S, RPS13 and TUB genes were the least appropriate reference genes (Table 2). During seed development, UCE, RPS13 and EF1α were the most appropriate reference genes according to the recommendations of ΔCt and NormFinder; by contrast, UBL, TEF2 and PLA were recommended by the BestKeeper algorithm, and the combination of RPS13 and RPII were recommended by geNorm. The comprehensive ranking order suggests that UCE, RPS13 and RPII were the optimal reference genes and that 18S, TUB and GAPDH were the least appropriate reference genes (Table 2).
For the entire growth cycle of Sacha inchi, the two most stable reference genes based on the ΔCt, BestKeeper, NormFinder and geNorm algorithms were UCE and ACT, PLA and UBL, ACT and EF1α, and the combination of ACT and UCE, respectively. The comprehensive ranking order showed that the top three most stable reference genes were UCE, ACT and EF1α (Table 2).
2.4. Reference Gene Validation
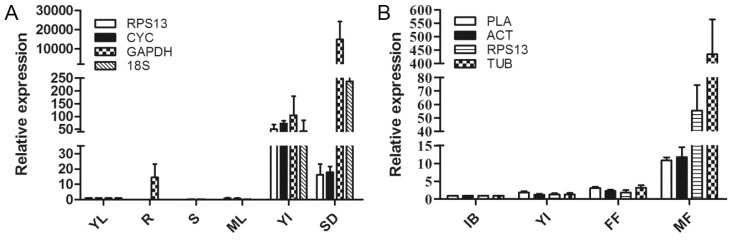
Sacha inchi AGAMOUS (PvoAG, GenBank GADC01013770), with homologs in other plants that are mainly expressed in floral organs [29,30,31], was chosen to further validate the reliability of the selected reference genes for the normalization of RT-qPCR data in Sacha inchi adult plants and flower developmental stages. The most stable reference genes identified for adult plants (RPS13 and CYC) and during flower development (PLA and ACT) were used as internal controls for data normalization. For comparison, the least stable reference genes identified in adult plants (GAPDH and 18S) and during flower development (RPS13 and TUB) were also considered. The results demonstrated that the expression patterns of PvoAG differed when using the most and least stable reference genes for normalization (Figure 3). In adult plants (Figure 3A), when the RPS13 and CYC genes were used for normalization, PvoAG was predominantly expressed in young inflorescences with relatively lower expression in seeds (90 DAP). However, the expression level of PvoAG in seeds (90 DAP) was substantially greater than in young inflorescences when using the least stable reference genes (GAPDH and 18S) for normalization. The PvoAG gene was also found to be expressed in mature roots when using GAPDH for normalization. During flower development (Figure 3B), when PLA and ACT were used for normalization, PvoAG was expressed in all developmental stages and at a higher level in male flowers. When RPS13 and TUB were considered, the expression pattern of PvoAG was similar to that obtained when using the most stable reference genes, but the expression level was over-estimated in male flowers. These findings suggest that the choice of reliable reference genes is essential for the accurate normalization of target gene expression levels.
Figure 3.
Relative quantification of the AGAMOUS homolog (PvoAG) in Sacha inchi using the validated reference genes for normalization. (A) PvoAG expression pattern in adult plants. The four kinds of bars indicate PvoAG expression levels normalized by different reference genes RPS13, CYC, GAPDH and 18S respectively; and (B) PvoAG expression pattern during flower development. The four kinds of bars indicate PvoAG expression levels normalized by different reference genes PLA, ACT, RPS13 and TUB respectively. YL, young leaf; R, root; S, stem; ML, mature leaf; YI, young inflorescence; SD, seed (90 DAP); IB, inflorescence bud; FF, female flower; MF, male flower.
3. Discussion
As a result of its high sensitivity, specificity and cost-efficiency, RT-qPCR has greatly improved the quality of measurements of expression levels of target genes in biological samples [32]. However, the accuracy of RT-qPCR analysis can be significantly affected by several factors, including RNA quality, the quantity of cDNA and the selection of reference genes [9,16]. To achieve high accuracy, a reference gene should have a relatively stable expression level in distinct biological samples, such as across tissues, developmental stages and experimental conditions. In this study, the expression stabilities of twelve candidate reference genes were estimated in various tissues and developmental stages of Sacha inchi. The UCE, ACT, and PLA genes were found to be the most stable genes in seedlings. For roots, stems, leaves, flowers, and seeds from adult plants, RPS13, CYC, and EF1α were recommended as reference genes for RT-qPCR. During the development of reproductive organs, PLA, ACT, and UCE were the optimal reference genes for flower development, whereas UCE, RPS13, and RPII were optimal for seed development.
In this study, four computational methods (ΔCt, BestKeeper, NormFinder and geNorm) were used to evaluate the stability of the expression levels of these twelve candidate reference genes. Here we found that the least stable genes computed by the four algorithms were almost the same, while the most stable genes differed. In the set of adult plant, the 18S gene was ranked last by all four algorithms, whereas the RPS13, the UCE, the EF1α and the combination of CYC and RPS13 genes were ranked first by ΔCt, BestKeeper, Normfiner and geNorm, respectively (Table 2). To obtain the most stable reference gene, we used the RefFinder tool that integrates the currently available major computational programs (ΔCt, BestKeeper, Normfinder and geNorm) to compare and rank the tested candidate reference genes. Based on the rankings from each above mentioned program, RefFinder assigned an appropriate weight to an individual gene and calculated the geometric mean of their weights for the overall final ranking. Accordingly, the RPS13 was recommended as the most appropriate reference gene in adult plant (Table 2).
The 18S ribosomal RNA is a component of the small subunit of eukaryotic ribosomes (40S). The 18S gene has been used as a reference gene for RT-qPCR normalization in many previous studies [33,34]. In Jatropha, the 18S was applied to normalize the expression of JcAOC and JcBD1 in various tissues under salt and cold stress conditions [35,36]. However, in this study, the 18S gene was the least stable gene across three experimental datasets, i.e., the adult plant, the seed developmental stage, and the entire growth cycle of Sacha inchi. The 18S gene has also been deemed inappropriate for gene expression analyses in Pisum sativum [37] and bamboo [20]. The GAPDH gene, which encodes a key enzyme involved in the glycolysis and gluconeogenesis [38], is another commonly used reference gene. It is the most stable reference gene in Jatropha over different tissues, developmental stages and experimental conditions [21]. And it has been also recommended in flax [39] and coffee [22,40]. However, GAPDH has been reported as the least stable reference gene in oil palm [23], peach [24], Petunia hybrida [25] and bamboo [20]. Similarly, in the present study, the GAPDH expression varied among tissues in Sacha inchi, except across the flower developmental stages in which it ranked the fourth. It is possible that GAPDH is not only a key enzyme involved in glycolysis but also participates in other processes. The TUB gene, which plays a crucial role in cell structural maintenance, has also been widely used as a reliable reference gene in switchgrass [41] and peach [24]. However, in our study, TUB was identified as a poor reference gene, similar to results for potato [42] and soybean [43]. Taken together, these results indicate that the most stable reference genes differ among plants or tissues. Hence, the choice of reference genes is very important.
Our results indicate that ACT is suitable for normalization in seedlings and during flower development in Sacha inchi. In Jatropha, ACT expression was more ubiquitous than in Sacha inchi, and was found across the different plant developmental stages and under cold-/drought-induced conditions [21]. UCE was ranked among the top three most stable reference genes for all tissues, with the exception that it was ranked fourth for the adult plant. Therefore, UCE is recommended for the normalization of gene expression in Sacha inchi. UCE has also been identified as one of the most stable reference genes in switchgrass [41], whereas UCE was the most variable reference gene for the tung tree [12] and Jatropha curcas [21]. In addition, we found that CYC was ranked first for the entire growth cycle of Sacha inchi and second for the adult plant in this study. The CYC gene was among the best reference genes for Petunia hybrida [37] and bamboo [24]. The RPS13 gene, which was used for the normalization of gene expression in Petunia hybrida [25], might also serve as a reliable reference gene for studies of adult plants, different developmental stages of seeds, and the entire growth cycle of Sacha inchi.
To illustrate the actual utility of validated reference genes in this study, the expression pattern of PvoAG was examined in Sacha inchi. AG belongs to the C-class genes in the ABC model of floral organ development [44]. In Arabidopsis, AG was mainly expressed in inflorescences and flowers, and was involved in the regulation of stamen and pistil development [44]. In poplar and strawberry, AG was also highly expressed in flowers with low levels in leaves, stems and seeds [36,45]. Here, in Sacha inchi adult plants, PvoAG was remarkably expressed in young inflorescences with relatively lower expression in seeds (90 DAP) when the most stable genes RPS13 and CYC were used for normalization (Figure 3A). This result is similar to the AG expressions in other plants mentioned above. However, when the least stable genes GAPDH and 18S were applied, the expression level of PvoAG was extremely high in seeds (90 DAP) (Figure 3A). Thus, these results further proved the necessity of selection of reliable reference genes in gene expression studies.
To date, numerous studies have reported that when evaluating levels of target gene expression, the results are more pronounced and reliable when two or more reference genes are utilized [42,46,47]. In this study, we have recommended the three most reliable reference genes for expression analyses of Sacha inchi for each of the aforementioned experimental conditions. The results of this study will help inform the selection of stable reference genes for future gene expression studies of Sacha inchi.
4. Experimental Section
4.1. Plant Materials
During the vegetative growth stage, tissues (roots, stems, young leaves and mature leaves) were collected from Sacha inchi (Plukenetia volubilis L.) seedlings that were grown in a growth chamber for three weeks after germination (12 h light/day, 25 °C). During the reproductive growth stage, tissues (roots, stems, young leaves, mature leaves, inflorescence buds, young inflorescences, female flowers, male flowers, and seeds at 15, 40, 90 and 130 DAP, respectively) were collected from one-year-old adult plants of Sacha inchi, which were grown in a field at the Xishuangbanna Tropical Botanical Garden (XTBG, 21°54′N, 101°46′E, 580 m in altitude) of the Chinese Academy of Sciences located in Mengla County, Yunnan Province, Southwest China [48]. The reproductive organs are shown in (Supplementary Figure S3). All of the tissues removed from plants were immediately frozen in liquid nitrogen and stored at −80 °C. Three biological replicates were collected for each sample.
4.2. Total RNA Extraction and cDNA Synthesis
Total RNA was isolated using the pBIOZOL Plant Total RNA Extraction Reagent according to the manufacturer’s instructions (BioFlux, Hangzhou, China). The RNA integrity was evaluated on a 2% agarose gel. The quantity and quality of the total RNA samples were assessed by measuring the absorbance ratio at 260/280 and 260/230 nm using a NanoDrop ND-1000 spectrophotometer (Thermo Scientific, Wilmington, DE, USA). Total RNA samples with A260/A280 and A260/A230 ratios greater than 1.8 were used for cDNA synthesis. An aliquot of total RNA (1 μg) was reverse transcribed using the PrimeScript™ RT reagent Kit with gDNA Eraser in a 20-μL reaction volume according to the manufacturer’s protocol (Perfect Real Time). All of the cDNA samples were diluted at 1:5 with RNase-free water and stored at −80 °C.
4.3. Selection of Candidate Reference Genes and Design of RT-qPCR Primers
Twelve Sacha inchi housekeeping genes (18S, ACT, CYC, EF1α, GAPDH, PLA, RPII, RPS13, TEF2, TUB, UBL and UCE) were selected as candidate reference genes. The cDNA sequences of these reference genes (Supplementary Figure S1) were obtained from the GenBank database (Available online: http://www.ncbi.nlm.nih.gov/nucleotide) and our RNA-seq transcriptome dataset of Sacha inchi. RT-qPCR primers (Table 1) were designed using Primer Premier 6 software [49] with the following parameters: melting temperature between 59 and 61 °C, primer length of 22–27 nucleotides, GC content of 40% to 60%, and PCR amplicon length of 101–202 bp.
4.4. RT-qPCR Conditions and Data Analysis
RT-qPCR was performed in a 96-well plate with a Roche LightCycler 480 real-time PCR detection system (Roche Diagnostics, Rotkreuz, Switzerland). The reaction was performed in a volume of 20 μL containing 1 μL of diluted cDNA, 10 μL of SYBR Premix Ex Taq™ II (Tli RNaseH Plus), and 0.25 μM of each primer. For each reference gene, no-template reactions were run as negative PCR controls. The cycling conditions were as follows: initial activation of 5 min at 95 °C; 45 cycles of 10 s at 95 °C, 20 s at 59 °C (60 °C for UCE); and 20 s at 72 °C. The specificity of the PCR amplicons was verified based on the melting curve from 60 to 95 °C. Each reaction was performed in three technical replicates with three biological replicates for each tissue. To calculate the gene-specific PCR efficiency, standard curves were generated from 10-fold serial dilutions of cDNA samples from young leaves for each primer pair. The values of the slopes and correlation coefficients were obtained from the standard curves. The corresponding PCR amplification efficiencies (E) were calculated according to the equation E = −1 + 10 (−1/slope) [50].
Gene expression stability was evaluated by applying four statistical algorithms: ΔCt [14], geNorm (version 3.5) [15], BestKeeper (version 1.0) [16], and NormFinder (version 0.953) [17]. The RT-qPCR data obtained from the Roche LightCycler 480 manager were exported into an Excel datasheet. Each statistical algorithm generates a measurement of reference gene stability that can be used to rank the stability order using RefFinder (Available online: http://omictools.com/reffinder-s2857.html) [51].
5. Conclusions
Twelve reference genes were evaluated in multiple tissues and during multiple developmental stages of flowers and seeds in Sacha inchi. The UCE, ACT and PLA genes were the most stable reference genes for seedlings of Sacha inchi, whereas the RPS13, CYC and EF1α genes were the most suitable reference genes for adult plants. The PLA, ACT and UCE genes are recommended as reference genes during flower development, and the UCE, RPS13 and RPII genes are recommended for studies during seed development. For analyses of the entire growth cycle of Sacha inchi, the three best reference genes are CYC, RPS13 and UCE.
Acknowledgments
This work was supported by the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-Z-15), and the Special Fund for the Technology innovation and Industrial Development Project of Yunnan Province (2012XB050), and the CAS 135 Program (XTBG-T02) to Zeng-Fu Xu. The authors thank the Central Laboratory of the Xishuangbanna Tropical Botanical Garden for providing the research facilities.
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/16/06/12513/s1.
Author Contributions
Longjian Niu and Zeng-Fu Xu designed research; Longjian Niu, Yan-Bin Tao, Mao-Sheng Chen, Qiantang Fu and Huiying He conducted research; Chaoqiong Li, Yuling Dong, Xiulan Wang and Zeng-Fu Xu analyzed data; Longjian Niu, Yan-Bin Tao and Zeng-Fu Xu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Gillespie L.J. A synopsis of neotropical Plukenetia (Euphorbiaceae) including two new species. Syst. Bot. 1993;18:575–592. doi: 10.2307/2419535. [DOI] [Google Scholar]
- 2.Gillespie L.J. A revision of paleotropical Plukenetia (Euphorbiaceae) including two new species from Madagascar. Syst. Bot. 2007;32:780–802. doi: 10.1600/036364407783390782. [DOI] [Google Scholar]
- 3.Krivankova B., Polesny Z., Lojka B., Lojkova J., Banout J., Preininger D. Sacha Inchi (Plukenetia Volubilis, Euphorbiaceae): A Promising Oilseed Crop from Peruvian Amazon; Proceedings of the Conference on International Agricultural Research and Development; Witzenhausen, Germany. 9–11 October 2007. [Google Scholar]
- 4.Fu Q., Niu L., Zhang Q., Pan B.-Z., He H., Xu Z.-F. Benzyladenine treatment promotes floral feminization and fruiting in a promising oilseed crop Plukenetia volubilis. Ind. Crop. Prod. 2014;59:295–298. doi: 10.1016/j.indcrop.2014.05.028. [DOI] [Google Scholar]
- 5.Zuleta E.C., Rios L.A., Benjumea P.N. Oxidative stability and cold flow behavior of palm, sacha-inchi, jatropha and castor oil biodiesel blends. Fuel Process Technol. 2012;102:96–101. doi: 10.1016/j.fuproc.2012.04.018. [DOI] [Google Scholar]
- 6.Wang X., Xu R., Wang R., Liu A. Transcriptome analysis of Sacha Inchi (Plukenetia volubilis L.) seeds at two developmental stages. BMC Genomics. 2012;13 doi: 10.1186/1471-2164-13-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bustin S. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): Trends and problems. J. Mol. Endocrinol. 2002;29:23–39. doi: 10.1677/jme.0.0290023. [DOI] [PubMed] [Google Scholar]
- 8.Nolan T., Hands R.E., Bustin S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- 9.Bustin S., Benes V., Nolan T., Pfaffl M. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005;34:597–601. doi: 10.1677/jme.1.01755. [DOI] [PubMed] [Google Scholar]
- 10.Udvardi M.K., Czechowski T., Scheible W.-R. Eleven golden rules of quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicot N., Hausman J.-F., Hoffmann L., Evers D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- 12.Han X., Lu M., Chen Y., Zhan Z., Cui Q., Wang Y. Selection of reliable reference genes for gene expression studies using real-time PCR in tung tree during seed development. PLoS ONE. 2012;7:e43084. doi: 10.1371/journal.pone.0043084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y., Chen D., Smith M.A., Zhang B., Pan X. Selection of reliable reference genes in Caenorhabditis elegans for analysis of nanotoxicity. PLoS ONE. 2012;7:e31849. doi: 10.1371/journal.pone.0031849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silver N., Best S., Jiang J., Thein S.L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 2006;7 doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vandesompele J., de Preter K., Pattyn F., Poppe B., van Roy N., de Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper-Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 17.Andersen C.L., Jensen J.L., Ørntoft T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 18.Brunner A.M., Yakovlev I.A., Strauss S.H. Validating internal controls for quantitative plant gene expression studies. BMC Plant Biol. 2004;4 doi: 10.1186/1471-2229-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chandna R., Augustine R., Bisht N.C. Evaluation of candidate reference genes for gene expression normalization in Brassica juncea using real time quantitative RT-PCR. PLoS ONE. 2012;7:e36918. doi: 10.1371/journal.pone.0036918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan C., Ma J., Guo Q., Li X., Wang H., Lu M. Selection of reference genes for quantitative real-time PCR in bamboo (Phyllostachys edulis) PLoS ONE. 2013;8:e56573. doi: 10.1371/journal.pone.0056573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L., He L.-L., Fu Q.-T., Xu Z.-F. Selection of reliable reference genes for gene expression studies in the biofuel plant Jatropha curcas using real-time quantitative PCR. Int. J. Mol. Sci. 2013;14:24338–24354. doi: 10.3390/ijms141224338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cruz F., Kalaoun S., Nobile P., Colombo C., Almeida J., Barros L.M., Romano E., Grossi-de-Sá M.F., Vaslin M., Alves-Ferreira M. Evaluation of coffee reference genes for relative expression studies by quantitative real-time RT-PCR. Mol. Breed. 2009;23:607–616. doi: 10.1007/s11032-009-9259-x. [DOI] [Google Scholar]
- 23.Yeap W.-C., Loo J.M., Wong Y.C., Kulaveerasingam H. Evaluation of suitable reference genes for qRT-PCR gene expression normalization in reproductive, vegetative tissues and during fruit development in oil palm. Plant Cell Tissue Organ. 2014;116:55–66. doi: 10.1007/s11240-013-0382-3. [DOI] [Google Scholar]
- 24.Tong Z., Gao Z., Wang F., Zhou J., Zhang Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol. Biol. 2009;10 doi: 10.1186/1471-2199-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallona I., Lischewski S., Weiss J., Hause B., Egea-Cortines M. Validation of reference genes for quantitative real-time PCR during leaf and flower development in Petunia hybrida. BMC Plant Biol. 2010;10 doi: 10.1186/1471-2229-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei L., Miao H., Zhao R., Han X., Zhang T., Zhang H. Identification and testing of reference genes for Sesame gene expression analysis by quantitative real-time PCR. Planta. 2013;237:873–889. doi: 10.1007/s00425-012-1805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi J., Yu S., Zhang F., Shen X., Zhao X., Yu Y., Zhang D. Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in Chinese cabbage (Brassica rapa L. ssp. pekinensis) Plant Mol. Biol. Rep. 2010;28:597–604. doi: 10.1007/s11105-010-0185-1. [DOI] [Google Scholar]
- 28.Zhu X., Li X., Chen W., Chen J., Lu W., Chen L., Fu D. Evaluation of new reference genes in papaya for accurate transcript normalization under different experimental conditions. PLoS ONE. 2012;7:e44405. doi: 10.1371/journal.pone.0044405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hou J.-H., Gao Z.-H., Zhang Z., Chen S.-M., Ando T., Zhang J.-Y., Wang X.-W. Isolation and characterization of an AGAMOUS homologue PmAG from the Japanese apricot (Prunus mume Sieb. et Zucc.) Plant Mol. Biol. Rep. 2011;29:473–480. doi: 10.1007/s11105-010-0248-3. [DOI] [Google Scholar]
- 30.Zhang J.Q., Li Z.N., Guo C., Liu G.F., Bao M.Z. Isolation and functional analyses of a putative floral homeotic c-function gene in a basal eudicot London plane tree (Platanus acerifolia) PLoS ONE. 2013;8:e63389. doi: 10.1371/journal.pone.0063389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanofsky M.F., Ma H., Bowman J.L., Drews G.N., Feldmann K.A., Meyerowitz E.M. The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature. 1990;346:35–39. doi: 10.1038/346035a0. [DOI] [PubMed] [Google Scholar]
- 32.Ginzinger D.G. Gene quantification using real-time quantitative PCR: An emerging technology hits the mainstream. Exp. Hematol. 2002;30:503–512. doi: 10.1016/S0301-472X(02)00806-8. [DOI] [PubMed] [Google Scholar]
- 33.Bustin S.A. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 34.Thellin O., Zorzi W., Lakaye B., de Borman B., Coumans B., Hennen G., Grisar T., Igout A., Heinen E. Housekeeping genes as internal standards: Use and limits. J. Biotechnol. 1999;75:291–295. doi: 10.1016/S0168-1656(99)00163-7. [DOI] [PubMed] [Google Scholar]
- 35.Liu B., Wang W.G., Gao J.H., Chen F., Wang S.H., Xu Y., Tang L., Jia Y.J. Molecular cloning and characterization of a jasmonate biosynthetic pathway gene for allene oxide cyclase from Jatropha curcas. Acta Physiol. Plant. 2010;32:531–539. doi: 10.1007/s11738-009-0430-0. [DOI] [Google Scholar]
- 36.Zhang B.Y., Su X.H., Zhou X.M. A MADS-box gene of Populus deltoides expressed during flower development and in vegetative organs. Tree Physiol. 2008;28:929–934. doi: 10.1093/treephys/28.6.929. [DOI] [PubMed] [Google Scholar]
- 37.Die J.V., Román B., Nadal S., González-Verdejo C.I. Evaluation of candidate reference genes for expression studies in Pisum sativum under different experimental conditions. Planta. 2010;232:145–153. doi: 10.1007/s00425-010-1158-1. [DOI] [PubMed] [Google Scholar]
- 38.Plaxton W.C. The organization and regulation of plant glycolysis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996;47:185–214. doi: 10.1146/annurev.arplant.47.1.185. [DOI] [PubMed] [Google Scholar]
- 39.Huis R., Hawkins S., Neutelings G. Selection of reference genes for quantitative gene expression normalization in flax (Linum usitatissimum L.) BMC Plant Biol. 2010;10:71. doi: 10.1186/1471-2229-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barsalobres-Cavallari C.F., Severino F.E., Maluf M.P., Maia I.G. Identification of suitable internal control genes for expression studies in Coffea arabica under different experimental conditions. BMC Mol. Biol. 2009;10 doi: 10.1186/1471-2199-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang L., Yan H., Jiang X., Zhang X., Zhang Y., Huang X., Zhang Y., Miao J., Xu B., Frazier T., et al. Evaluation of candidate reference genes for normalization of quantitative RT-PCR in switchgrass under various abiotic stress conditions. BioEnerg. Res. 2014;7:1201–1211. doi: 10.1007/s12155-014-9457-1. [DOI] [Google Scholar]
- 42.Reid K., Olsson N., Schlosser J., Peng F., Lund S. An optimized grapevine RNA isolation procedure and statistical determination of reference genes for real-time RT-PCR during berry development. BMC Plant Biol. 2006;6 doi: 10.1186/1471-2229-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu R., Fan C., Li H., Zhang Q., Fu Y.-F. Evaluation of putative reference genes for gene expression normalization in soybean by quantitative real-time RT-PCR. BMC Mol. Biol. 2009;10 doi: 10.1186/1471-2199-10-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizukami Y., Ma H. Ectopic expression of the floral homeotic gene AGAMOUS in transgenic Arabidopsis plants alters floral organ identity. Cell. 1992;71:119–131. doi: 10.1016/0092-8674(92)90271-D. [DOI] [PubMed] [Google Scholar]
- 45.Rosin F.M., Aharoni A., Salentijn E.M., Schaart J.G., Boone M.J., Hannapel D.J. Expression patterns of a putative homolog of AGAMOUS, STAG1, from strawberry. Plant Sci. 2003;165:959–968. doi: 10.1016/S0168-9452(03)00233-4. [DOI] [Google Scholar]
- 46.Gutierrez L., Mauriat M., Guénin S., Pelloux J., Lefebvre J.-F., Louvet R., Rusterucci C., Moritz T., Guerineau F., Bellini C., et al. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008;6:609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 47.Exposito-Rodriguez M., Borges A., Borges-Perez A., Perez J. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biol. 2008;8 doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Niu L., Li J., Chen M.-S., Xu Z.-F. Determination of oil contents in Sacha inchi (Plukenetia volubilis) seeds at different developmental stages by two methods: Soxhlet extraction and time-domain nuclear magnetic resonance. Ind. Crop. Prod. 2014;56:187–190. doi: 10.1016/j.indcrop.2014.03.007. [DOI] [Google Scholar]
- 49.Wang J.H., Ni Z.H., Duan Z.P., Wang G.Q., Li F. Altered expression of hypoxia-inducible factor-1 alpha (Hif-1α) and its regulatory genes in gastric cancer tissues. PLoS ONE. 2014;9:e99835. doi: 10.1371/journal.pone.0099835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramakers C., Ruijter J.M., Deprez R.H.L., Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- 51.Hao X., Horvath D.P., Chao W.S., Yang Y., Wang X., Xiao B. Identification and evaluation of reliable reference genes for quantitative real-time PCR analysis in tea plant (Camellia sinensis (L.) O. Kuntze) Int. J. Mol. Sci. 2014;15:22155–22172. doi: 10.3390/ijms151222155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.