Abstract
A bioflocculant named MBF-UFH produced by a Bacillus species isolated from sediment samples of Algoa Bay of the Eastern Cape Province of South Africa was characterized. The bacterial identification was through 16S rDNA sequencing; nucleotide sequences were deposited in GenBank as Bacillus sp. AEMREG7 with Accession Number KP659187. The production of the bioflocculant was observed to be closely associated with cell growth. The bioflocculant had the highest flocculating activity of 83.2% after 72 h of cultivation, and approximately 1.6 g of purified MBF-UFH was recovered from 1 L of fermentation broth. Its chemical analyses indicated that it is a glycoprotein composed of polysaccharide (76%) and protein (14%). Fourier transform infrared spectroscopy (FTIR) revealed that it consisted of hydroxyl, amide, carboxyl and methoxyl as the functional moieties. Scanning electron microscopy (SEM) revealed the amorphous structure of MBF-UFH and flocculated kaolin clay particles. The maximum flocculating activity of 92.6% against kaolin clay suspension was achieved at 0.3 mg/mL over pH ranges of 3–11 with the peak flocculating rate at pH 8 in the presence of MgCl2. The bioflocculant retained high flocculating activity of 90% after heating at 100 °C for 1 h. MBF-UFH appears to have immense potential as an alternative to conventional chemical flocculants.
Keywords: Bacillus sp. AEMREG7, MBF-UFH, flocculating activity, glycoprotein, thermostable
1. Introduction
The flocculation stage is a major phase in water treatment technology for the exclusion of both organic and inorganic pollutants [1]. Flocculants are substances used in the clumping of colloids, cells and suspended solids into larger sizeable flocs that can be removed effectively from solution by sedimentation [2]. Applications of flocculants have included downstream processes in the fermentation industries, as well as drinking and waste-water treatment facilities [3]. Flocculants may be categorized into three groups: organic flocculants, such as polyacrylamide derivatives; inorganic flocculants, such as polyaluminum chloride and ferric chloride; naturally-occurring flocculants, such as chitosan, sodium alginate and bioflocculants. However, the choice of flocculants has a major influence on the performance of the flocculation process [4].
Bioflocculants stands out among others, as they have the advantages of innocuousness, biocompatibility, biodegradability and environmentally friendliness, unlike organic and inorganic flocculants, which are toxic and whose degradation intermediates are difficult to remove from the environment [5]. Besides, organic flocculants, such as polyacrylamide and polyethylene imine derivatives, have been implicated in adverse human health effects [6]. An outstanding example is aluminum salts, which have been demonstrated to cause Alzheimer’s disease in humans [7]. Conversely, the enormous advantages associated with bioflocculants motivate its consideration as an alternative, hence the vast interest in the scientific and industrial community worldwide [8].
Bioflocculants are mostly composed of macromolecular substances, such as polysaccharides, protein, lipids and nucleic acids [9]. The chemical composition and flocculating efficiency of bioflocculants depends on some factors, including the nature of the environment in which bioflocculant-producing microorganisms are isolated, the media compositions in which the microorganisms are cultivated, the functional groups and molecular weight of the bioflocculant [10]. Several studies have demonstrated the efficiencies of bioflocculants in the treatment of drinking/waste waters and other downstream processing [6].
Nevertheless, low flocculating efficiency, low yields and high cost of production compared with the conventional flocculants are major limitations to large-scale production and application of bioflocculants [11,12,13]. Consequently, there is a need for continual exploration for novel microbes with efficient bioflocculant-producing capability. Thus, efficient bioflocculant-producing microbes should possess bioflocculants with high flocculating efficiency and yields [14,15].
Marine environment have remained a potential reservoir of microbes with novel metabolites, and the exploitation of this ecosystem through prospecting for microbes with novel metabolites is still very nascent [16,17,18,19,20]. Consequently, the current study aimed at characterizing the bioflocculant produced by Bacillus sp. AEMREG7 isolated from a sediment sample of Algoa Bay in the Eastern Cape Province of South Africa.
2. Results and Discussion
2.1. Time Course of Bioflocculant Production
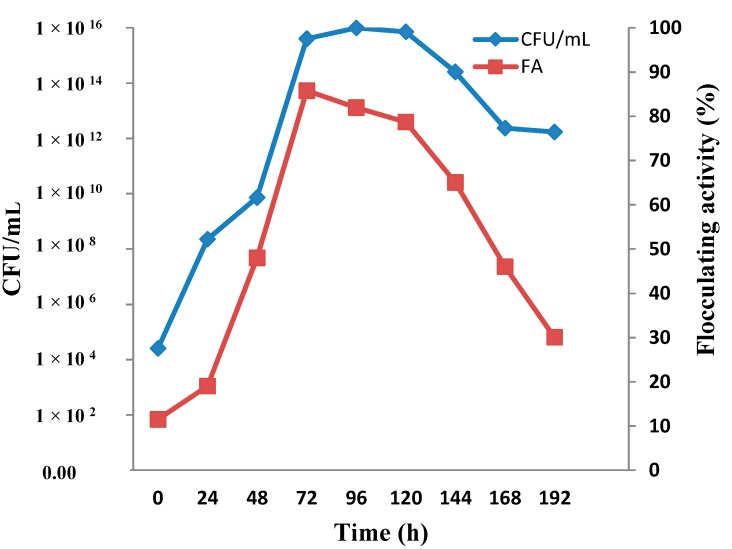
Figure 1 shows the relationship between MBF-UFH production and cell growth over a cultivation time of 192 h. Most microorganisms produced bioflocculant during the exponential growth phase [12]. The flocculating activity of the bioflocculant increased gradually with an increase in cultivation time. Generally, the production of MBF-UFH by Bacillus sp. AEMREG7 was observed to correspond with cell growth. Nonetheless, the highest flocculating activity of 83% was attained at 72 h in the late exponential growth phase, thus indicating that the production of MBF-UFH was closely associated with cell growth [21]. However, the decrease in the flocculating activity observed after 72 h may be due to the deflocculating enzyme released by the microorganism during the death phase of the cells. These results were similar to the findings of Wu and Ye [22] in which the bioflocculant production was synchronous with cell growth and reached maximum flocculating activity in the late logarithmic growth phase and early stationary phase. Furthermore, an increase in cultivation time led to a decrease in bioflocculant production as the flocculating activity decreased steadily. This decrease in flocculating activity of MBF-UFH might be due to deflocculating enzymatic activities or accumulation of toxic metabolic wastes affecting the produced bioflocculant [9]. Therefore, a cultivation time of 72 h was chosen for the subsequent experiments. It is apparent that MBF-UFH biosynthesis occurred during different microbial growth phases for different organisms [23]. Additionally, under optimum culture conditions, about 1.6 g of purified MBF-UFH was recovered from 1 L of fermentation broth of Bacillus sp. AEMREG7, which is higher than 0.4 g of the purified bioflocculant produced by Aspergillus flavus [24]. On the contrary, the bioflocculant production by Chryseobacterium daeguense W6 was released into the culture broth in the death phase of cell growth, when the nutrient in the medium had been depleted [10].
Figure 1.
Time course of bioflocculant production by Bacillus sp. AEMREG7.
2.2. Fourier Transform Infrared Analysis
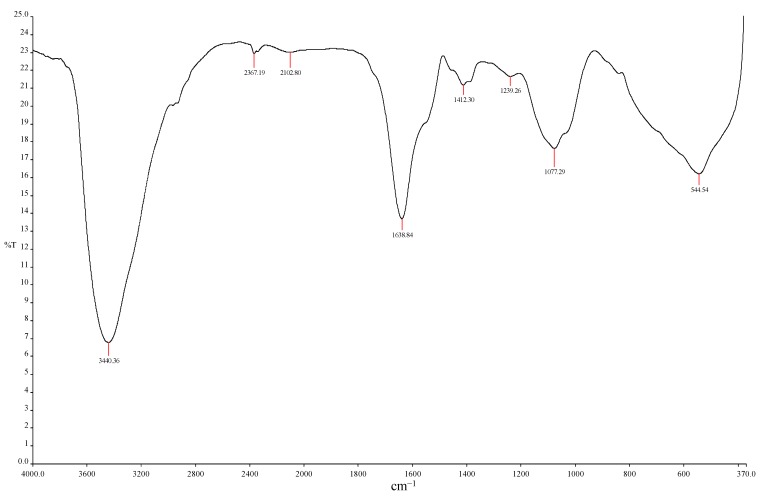
The functional moieties in the molecular chain of MBF-UFH were identified with FTIR spectrophotometry (Figure 2). The spectrum displaced an intense broad stretching peak at 3440 cm−1, which indicated the presence of a hydroxyl or amide group [25,26]. The water solubility of the bioflocculant was attributed to the presence of hydroxyl group forming a hydrogen bond with a water molecule. A weak peak observed at 2367 cm−1 was either CO2 adsorption or may be from the amine group [27]. Furthermore, an asymmetric stretching peak was at 1638 cm−1, which showed the presence of carbonyl group stretching vibration in the peptide [28]. The peak detected at 1412 cm−1 could be ascribed to the symmetric stretching of the –COO− group [18]. The presence of carboxyl groups provides more adsorption sites for particle attachment, so many particles can be adsorbed to the long molecular chain of the bioflocculant [29]. The absorption peaks ranging from 1000–1200 cm−1 were designated to C–O–C and C–O, which indicated the presence of polysaccharides [30,31]. The peak at 1077 cm−1 indicated the presence of methoxyl groups [9]. The characteristics of the FTIR spectrum showed the presence of hydroxyl, amide, carboxyl and methoxyl groups in the MBF-UFH structure as the main functional moieties preferred for flocculation [32].
Figure 2.
Fourier transform infrared (FTIR) spectra of purified MBF-UFH produced by Bacillus sp. AEMREG7.
2.3. Scanning Electron Microscopy Imaging
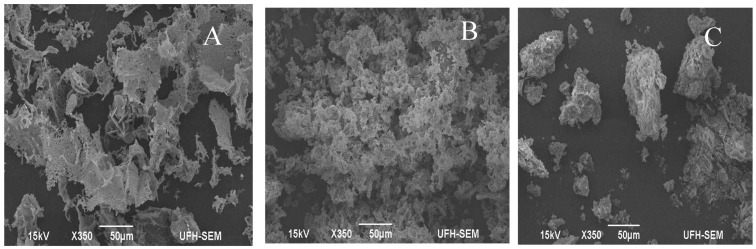
Surface morphology structure of the bioflocculant was explicated by scanning electron microscopy (SEM). SEM is a type of electron microscope that divulges the image of a sample by scanning it with a high-energy beam of electrons in a raster scan pattern [25]. The electrons interrelate with the atoms that make up the sample, producing signals that contain information about the sample’s surface structure [33]. The SEM image showed that MBF-UFH has an amorphous structure of a compact nature (Figure 3A). The configuration of this bioflocculant may be accountable for its high flocculation efficiency. Before the flocculation process, the kaolin clay particles appeared to be fine and scattered (Figure 3B), and after the flocculation process, the functional moieties in the molecular chain of MBF-UFH were used for attachment on the kaolin clay particle. Consequently, the interaction between the bioflocculant and kaolin clay particle resulted in the formation of flocs that later aggregated to larger sized flocs, which precipitated out of the suspension as the result of gravity (Figure 3C). This observation showed that bridging played a vital role in the flocculation process [34]. These results concur with previous findings for the purified bioflocculants produced by a consortium of Streptomyces and Cellulomonas species and Nocardiopsis aegyptia sp. nov. [6,17].
Figure 3.
SEM imaging of purified MBF-UFH produced by Bacillus sp. AEMREG7 (A), kaolin clay particles (B) and kaolin clay suspension flocculated with MBF-UFH (C).
2.4. Elemental Analysis by Energy Dispersive X-ray Spectroscopy (EDX)
The EDX analysis of the purified MBF-UFH revealed its elemental composition in mass proportion (% w/w): C (17.21), N (6.66), O (40.04), Na (5.21), Mg (5.02), P (7.90), S (0.60), Cl (6.11), K (1.63) and Ca (9.63). Likewise, the presence of non-sugar components is usually small; nevertheless, they may provide flexibility and stabilize MBF-UFH [6]. In agreement with our observation, Singh et al. [35] documented that the exopolysaccharide produced by Bacillus licheniformis was composed of the following elements in mass proportion (% w/w): C (38.48), O (55.71), P (0.50), S (1.47), Cl (1.24), Ca (0.25), Na (2.34). Furthermore, Mabrouk [17] reported analogous cases with the bioflocculant produced by Nocardiopsis aegyptia sp. nov., which was composed of the following elements in mass proportion (% w/w): Na (7), P (6.9), S (1.9), Cl (66.3), K (16.9), Cu (0.5), Zn (0.6).
2.5. Physicochemical Properties of Purified Bioflocculant
2.5.1. MBF-UFH Dosage
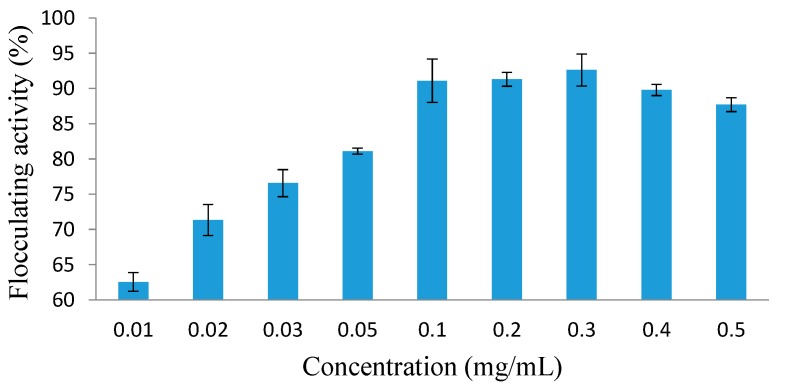
The effect of MBF-UFH dosage on flocculation efficiency for kaolin clay suspension was examined in an attempt to determine the most cost-effective dose for flocculation process. Under optimal conditions, the maximum flocculating activity is usually attained at the optimal bioflocculant dosage [36]. The flocculating activity of purified MBF-UFH was investigated in a range of 0.01–0.5 mg/mL (Figure 4). The flocculating activity of 61.5% was achieved at 0.01 mg/mL, and a further increase in MBF-UFH dosage resulted into a gradual decrease in flocculating activity. Nevertheless, the optimum bioflocculant dosage range for effective flocculation efficiency of over 90% was observed between 0.1 and 0.3 mg/mL, with the highest flocculating activity of 92.6% attained at 0.3 mg/mL. However, there was no significant increase in the flocculating activity of MBF-UFH when the dosage was increased from 0.1 up to 0.3 mg/mL (Figure 4). Although, the flocculating activity of MBF-UFH was low at 0.01 mg/mL (61.5%) compared to the flocculating activity noticed in the optimum range of 0.1–0.3 mg/mL (above 90%). At a lower dosage, MBF-UFH was relatively small to destabilize the negative charge of the kaolin clay particles, and the excess kaolin particles restabilized and increased the turbidity of the suspension; lower flocculating activity was noted in comparison to the flocculating rate observed at 0.1 mg/mL (Figure 4). This showed that the bridging effect of MBF-UFH was lower at 0.01 mg/mL compared to when it was at a higher dosage. On the contrary, the flocculating activity slightly decreased to 87.7% on increasing MBF-UFH dosage to 0.5 mg/mL compared with the flocculating activity of over 90% observed at an optimum dosage range between 0.1 and 0.3 mg/mL. This observation was in agreement with those reported elsewhere [37,38,39]. The decrease in flocculating activity of MBF-UFH observed at 0.5 mg/mL might be due to the over addition of the negatively-charged MBF-UFH, generating strong repulsive forces between the kaolin clay particles and the bioflocculant. These processes restabilized the suspended particles, increasing the viscosity of the suspension, blocking the adsorption sites and noticeably reduced floc formation [40]. These findings are consistence with previous studies reported by Elkady et al. [41] and Zheng et al. [9]. It has been extensively documented that a lower concentration of bioflocculants with a high flocculating efficiency will contribute to treatment cost reduction. Besides, information on the dosage requirement is substantial for future prospects in water treatment applications [28].
Figure 4.
Effect of MBF-UFH concentration on the flocculating activity of purified bioflocculant produced by Bacillus sp. AEMREG7.
2.5.2. Effect of Cations on the Flocculating Activity
In order to examine the effect of cations on the flocculating activity of MBF-UFH, a 4 g/L kaolin suspension in distilled water was used as the test material. Flocculation assays were set up in which CaCl2 was replaced by other cations when necessary, and the synergistic effects of cations were found in all of the cations investigated, except for Fe3+ (Table 1). It was observed that oppositely-charged cations reduced the particle surface charge density, so that the MBF-UFH molecules and kaolin particles could draw closer to each other, and the attractive forces were capable of overcoming the electrostatic repulsion to become effective. This interaction brought about the flocculation process of MBF-UFH. Factors influencing the effects of cations on flocculation vary; this may include surface charge capacity and the pH of the reaction mixture [3,37]. The studied bioflocculant alone flocculated kaolin clay suspension over 70% without the addition of cations. Prior to this study, very few bioflocculants have been reported to have a high flocculation rate without cations’ aid [39]. The synergistic effect of cations was observed most with monovalent and divalent cations, showing substantial enhancement on flocculating activity (with over 20%), with the highest flocculating rate observed with Mg2+ (Table 1). The flocculating activity of MBF-UFH was inhibited by Fe3+. However, a variation in flocculating activity was observed with Al3+, while Fe3+ may be accounted for by the surface charge availability of these cations in relation to the charge distributions of the bioflocculants. The trivalent cation could change the surface charge of kaolin particles and cover the adsorption sites, which led to a decrease in flocculating activity noticed in the presence of Al3+. Nonetheless, another possible scenario may be due to the effect of cation on the ionic strength of the suspension, which might also affect the conformations of MBF-UFH chain. Since MBF-UFH showed a flocculation rate at a lower dosage, it showed that bridging was substantially involved in the flocculation process. However, bridging flocculation requires that the MBF-UFH chain remains in an extended conformation; a higher charge density or higher proportion of charged groups favored the presence of the extended conformation of MBF-UFH chain, provided that all of the groups are either anionic or cationic by means of the electrostatic repulsion. Replacing divalent cations by trivalent cations increases notably the ionic strength of the suspension, and therefore, the electrostatic repulsive forces among charged groups of the bioflocculant chain decrease notably. This fact causes MBF-UFH chain conformation changes towards a more compact conformation, which is not able to form bridges. This might probably be the main cause of the poor flocculation ability of MBF-UFH in the presence of Al3+ and Fe3+. In addition, the synergistic effect of Al3+ with MBF-UFH and the antagonistic effect of Fe3+ with MBF-UFH thus lead to high flocculating activity for the Al3+ and lower flocculating activity in the presence of Fe3+ when the synergistic effects of trivalent cations on the flocculating activity of MBF-UFH were compared. There have been several reports on divalent cations stimulating the flocculating activity of bioflocculants [8,22]. Ostensibly, it seems that the monovalent and divalent cations help to neutralize negative charges on MBF-UFH and the suspended kaolin particles, shortening the distance between them, increasing the initial adsorption of MBF-UFH onto the kaolin particle and thus leading to floc formation and sedimentation [22,42]. It has been well documented that the addition of cations to suspensions is necessary to induce the effective flocculation capability of a bioflocculant; the effects of cations on the flocculating activity of MBF-UFH are similar to the previous studies by Okaiyeto et al. [43] on the bioflocculant produced by Micrococcus sp. Okoh. On the other hand, the bioflocculants produced by Aspergillus flavus and Klebsiella pneumonia were cation independent, which showed an outstanding performance in kaolin clay suspension without the addition of metal ions [24,39].
Table 1.
Effect of cations on the flocculating activity of purified MBF-UFH
| Cation | Na+ | K+ | Li+ | Mg2+ | Ca2+ | Mn2+ | Fe3+ | Al3+ | BA |
|---|---|---|---|---|---|---|---|---|---|
| FA | 93.29 | 92.18 | 92.46 | 94.77 | 91.13 | 92.68 | 9.90 | 68.36 | 70.94 |
| (%) ±SD | 1.47 | 1.49 | 1.54 | 0.71 | 2.35 | 1.12 | 5.14 | 2.51 | 3.12 |
FA, flocculating activity; BA-Bioflocculant (MBF-UFH) alone.
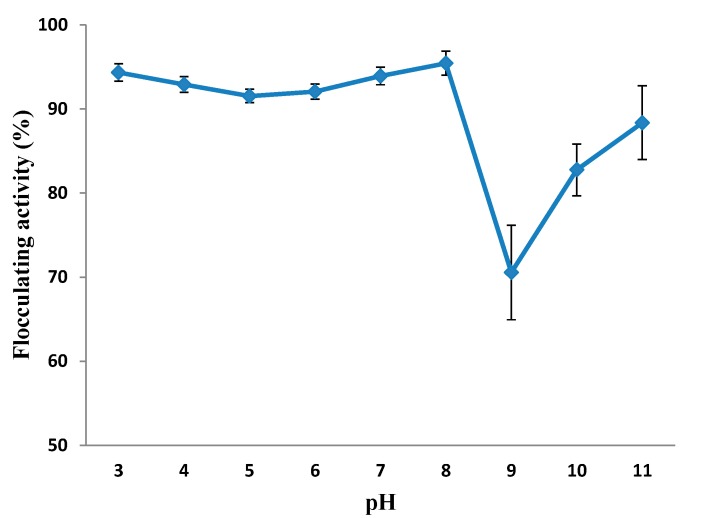
2.5.3. Effect of pH on the Flocculating Activity of Purified MBF-UFH
The pH of reaction mixtures is a key factor influencing the flocculation process [38]. One of the ways in which pH influences flocculating activity is by affecting the stability of suspended particles and floc formation [44]; the effect of pH on the flocculating activity of purified MBF-UFH was evaluated, and the results are depicted in Figure 5. The bioflocculant had a strong flocculating activity over a wide range of pH, 3–11. There was no significant difference in the flocculating activity of MBF-UFH between the pH ranges 3–8. However, the peak flocculating activity of 95.4% was achieved at weak alkaline condition, pH 8, and a sharp decline in flocculating activity was noticed at pH 9, which might be due to the fact that the surface charge spatial arrangement is both pH and temperature dependent. Thus, it would be safe to assume that the spatial charge arrangements for flocculation were not ambient at pH 9 and within the alkaline range. MBF-UFH was tolerant to the extreme pH and showed excellent flocculating activity in a strong acidic than basic condition. Hence, this suggested that MBF-UFH could be applied under acidic, neutral and alkaline circumstances, as it shows different electric states at different pH, which, in turn, influences the bridging efficiency of MBF-UFH for kaolin clay particles [38,45]. These observations are similar to the findings reported by Ugbenyen et al. [44] in which the flocculating activity of the bioflocculant produced by a consortium of Cobetia and Bacillus species was over 70% across a wide pH range of 3–11 with the highest flocculating activity attained at pH 8. The flocculating activity of the bioflocculant produced by Bacillus sp. F19 reached the maximum at pH 2 and maintained excellent flocculating activity within a range of pH 2–9. On the other hand, the bioflocculant produced by Ruditapes philippinarum flocculated over a wide pH range with the optimum flocculation rate between pH 7 and 9 [45].
Figure 5.
Effect of pH on the flocculating activity of purified MBF-UFH produced by Bacillus sp. AEMREG7.
2.5.4. Thermal Stability of Purified MBF-UFH
The thermal stability of the purified bioflocculant was examined at 50, 60, 70, 80, 90 and 100 °C for 1 h at each temperature. As depicted in Figure 6, MBF-UFH had an exceptional flocculating activity of 90% over all of the temperature regimes. The high flocculating efficiency showed by MBF-UFH at 100 °C indicated that it is thermally stable and consisted predominantly of polysaccharide [46,47]. The thermal stability may be due to the presence of a hydroxyl group involved in the formation of hydrogen bonds in MBF-UFH structure [44]. The stability of MBF-UFH was similar to the bioflocculant produced by C. glutamicum, which retained high flocculating activity of 96.9% at 80 °C, but the stability decreased slightly on increasing the temperature to 100 °C [47]. Besides, the bioflocculant produced by Aspergillus flavus was thermally stable, which retained high flocculating activity above 90% over a temperature range of 10–100 °C [24]. Nevertheless, Salehizadel et al. [48] reported a less stable bioflocculant that lost about 50% of the flocculating activity after heating for 15 min at 100 °C.
Figure 6.
Thermal stability of purified MBF-UFH produced by Bacillus sp. AEMREG7.
2.6. Comparison of Flocculating Activity of MBF-UFH with Conventional Flocculants
The flocculating efficiency of MBF-UFH for kaolin clay suspension was compared with the conventional flocculants, such as aluminum chloride, ferric chloride and anionic polyacrylamide (Table 2). The maximum flocculation rate was achieved at the optimum concentration of these flocculants; and it was observed that polyacrylamide has the highest flocculating efficiency (94.30%), followed by MBF-UFH (91.1%), aluminum chloride (67.99%) and ferric chloride (42.78%). These findings showed that MBF-UFH was significantly more efficient than both aluminum chloride and FeCl3, but slightly less efficient than polyacrylamide. The results suggest the great potential of MBF-UFH as an alternative to the commonly-used flocculants in waste/drinking water treatment.
Table 2.
Comparison of flocculating activity of MBF-UFH with conventional flocculants.
| Flocculant | Conc. (mg/mL) | FA (%) |
|---|---|---|
| Aluminum chloride | 1 | 67.99 |
| FeCl3 | 1 | 42.78 |
| Polyacrylamide | 0.1 | 94.30 |
| Bioflocculant | 0.1 | 91.1 |
Conc., concentration.
2.7. Proposed Flocculation Mechanism of MBF-UFH
The presence of a hydroxyl group in the molecular chain of MBF-UFH suggested that ionic bonds might be responsible for the interaction between MBF-UFH molecules and the kaolin clay particle, which led to the high flocculating efficiency observed in our study [33]. The force of adsorption may have likely come from hydrogen bonds that were formed between OH groups of MBF-UFH and the kaolin clay particles. Besides, the presence of the hydroxyl group might also be responsible for the high solubility property of the bioflocculant. In addition, its thermal stability could be linked to the presence of hydroxyl group involved in the formation of hydrogen bonds in the MBF-UFH structure [44]. Likewise, the presence of carboxyl groups in MBF-UFH indicated that there were chemical bonds existing between the COO− groups and kaolin clay particles. The carboxyl groups in MBF-UFH molecules were used as binding sites for the kaolin particle mediated by Mg2+. Therefore, we proposed that the flocculation process might be by bridging and charge neutralization.
Bridging was proposed to be involved in the flocculation of MBF-UFH, since a high flocculating activity of 62.5% was observed even at a lower dosage of 0.01 mg/mL. Bridging occurred after the cation-kaolin complexes adsorbed onto the bioflocculant chains, leading to the formation of three-dimensional flocs, which were capable of rapid setting [22]. The MBF-UFH molecule extends the functional moieties beyond the particle’s surface to the solution and attracts other particles in solution, thereby resulting in the aggregation of bigger flocs that can easily precipitate out of solution as a result of the bridging effect, and this phenomenon was confirmed by the SEM observations.
The role of cations is to increase the adsorption of MBF-UFH molecules on the surface of suspended particles by decreasing the negative charge on both MBF-UFH and the kaolin particle. When Mg2+ was added to the kaolin suspension, a decrease in charge density occurs, which eventually reversed the negatively-charged particles to positive and, hence, led to inter-particle bridging between kaolin particles and MBF-UFH. The divalent cation Mg2+ weakened the electrostatic repulsive forces between the bioflocculant and the kaolin clay particles; shortening the distance between them by compressing the double layer of kaolin particles and increasing the initial adsorption of MBF-UFH on the kaolin clay particles. Thus, electrostatic happened to be the main acting force between bioflocculant and the kaolin particles. Consequently, the functional moieties in the MBF-UFH molecule (hydroxyl, carboxyl and amine) adsorbed the kaolin clay particles and formed hydrogen bonds with them. Thus, the negatively-charged carboxyl group (COO−) of MBF-UFH could react with the positively-charged site of the suspended kaolin particles. It can be assumed that cations stimulate flocculation by neutralization and stabilization of residual negative charges of the carboxyl group of the bioflocculant MBF-UFH forming a bridge that binds kaolin particles to each other [22].
Moreover, since the chemical composition analyses revealed that MBF-UFH is composed of both polysaccharide and protein, this showed that the flocculation process might involve multiple functional moieties from both polysaccharide and protein. Multiple functional moieties imply many adsorption sites for the kaolin particles, which led to the high flocculating efficiency observed with MBF-UFH [49]. Furthermore, it was observed that MBF-UFH alone without cation flocculated kaolin clay suspension above 70%; this showed that the flocculation process could also be through charge neutralization as a result of electrostatic attraction between the positively-charged amino group of the protein part of MBF-UFH and the negative charge on the kaolin clay particle [50].
3. Experimental Section
3.1. Microorganism and Culture Conditions
Bacillus sp. AEMREG7 was previously isolated from a sediment sample of Algoa Bay in the Eastern Cape Province of South Africa, identified by 16S rDNA sequencing and preserved in 20% glycerol stock at −80 °C as part of the culture collection of the Applied and Environmental Microbiology Research Group (AEMREG), University of Fort Hare, South Africa. The activation medium was composed of 10 g tryptone, 3 g beef extract and 5 g NaCl in 1 L of filtered seawater [51]. The medium was sterile by autoclaving at 121 °C for 30 min and allowed to cool down at room temperature. The bacteria was activated by inoculating 5 µL of the glycerol stock into 5 mL of the activation medium and incubated overnight at 28 °C on a rotary shaker at 160 rpm. The growth medium was composed of 20 g glucose, 5 g K2HPO4, 2 g KH2PO4, 0.3 g (NH4)2SO4, 0.5 g urea, 0.5 g yeast extract, 0.3 g MgSO4·7H2O, 0.1 g NaCl in 1 L of filtered seawater [52]. After 24 h of fermentation, 2 mL of the fermented broth were inoculated into 50 mL of sterile growth medium incubated on rotary shaker at 160 rpm, 28 °C for 72 h. The flocculating activity of the cell-free supernatant was determined by flocculation assay after separating the bacterial cells from the culture broth by centrifugation at 4000 rpm for 30 min.
3.2. Determination of Flocculating Activity
A kaolin clay suspension was used as a model for the natural turbidity of raw surface water to determine the flocculating activity of the bioflocculant produced by Bacillus sp. AEMREG7. Four grams of kaolin clay (Merck, Darmstadt, Germany) were suspended in 1 L of distilled water to make the kaolin suspension. Two milliliters of cell-free supernatant were added to 100 mL of kaolin suspension in 250-mL conical flask containing 3 mL of CaCl2 (1% w/v). The mixture was agitated for 60 s, transferred to a 100-mL gradual measuring cylinder and allowed to stand for 5 min. The optical density (OD) of the supernatant was measured using a spectrophotometer (Helios Epsilon, USA) at 550 nm. The control experiment was set up by replacing the bioflocculant with 2 mL of freshly-prepared un-inoculated medium. The flocculating efficiency was calculated with the following equation:
| Flocculating Efficiency (%) = (A − B/A) × 100% |
where A and B are optical densities of the control and sample read at 550 nm, respectively.
3.3. Time Course of MBF-UFH Production
The time course of MBF-UFH production was done according the method described by Liu et al. [53] and Nwodo et al. [18] with some modifications. The culture broth (24 h old) was adjusted to OD660 0.1; then, the standardized culture broth was inoculated into 200 mL of fermentation medium contained in 500 mL flasks. The flasks were placed in a rotary shaker at 160 rpm, 28 °C for 192 h. The production of MBF-UFH was monitored over time by withdrawing 10 mL of the culture broth at intervals of 24 h for flocculating activity determination and bacterial cell growth via viable cell count (CFU/mL).
3.4. Bioflocculant Purification
The purification of MBF-UFH was carried out according to the description of Cosa et al. [54] and Li et al. [55] and with some modifications. After 72 h of fermentation, the culture broth was centrifuged at 4000 rpm for 30 min at 15 °C. The viscosity of the culture broth was reduced by diluting with two volumes of distilled water and then centrifuged at 4000 rpm for 30 min in order to remove bacterial cells. The pellet was discarded, and two volumes of cold ethanol were added to the supernatant and kept at 4 °C overnight for ethanol precipitation. The resulting precipitate was collected by centrifugation at 4000 rpm for 30 min at 15 °C and lyophilized to obtain crude MBF-UFH. The crude bioflocculant was dissolved in 100 mL of distilled water, to which one volume of a mixture of chloroform and n-butyl alcohol (5:2 v/v) was added, stirred for 60 s and kept overnight at room temperature. The upper phase was collected by centrifugation at 4000 rpm for 30 min at 15 °C, vacuum-dried and re-dissolved in distilled water. MBF-UFH solution was dialyzed against de-ionized water overnight and then lyophilized to obtain a purified MBF-UFH.
3.5. Chemical Composition Analysis of a Purified MBF-UFH
The total sugar content of MBF-UFH was determined according to the phenol-sulfuric method described by Chaplin and Kennedy [56] using glucose as the standard. The total protein contain was determined by the Bradford method described by Bradford [57] using bovine serum albumin (BSA) as the standard. The functional moieties in the MBF-UFH molecule were identified using an FTIR analyzer (Perkin Elmer System 2000, Buckinghamshire, UK). Dried MBF-UFH sample was ground with KBr powder and pressed into pellets for FTIR spectra measurement in the frequency range of 4000−400 cm−1 [33]. The surface morphology of MBF-UFH and kaolin clay particles was observed and elucidated using scanning electron microscopy (SEM) JEOL (JSM-6390LV, Tokyo, Japan). Five milligrams of MBF-UFH, kaolin clay and dried flocculated kaolin suspension were added on slides separately and fixed by air-drying. The fixed specimens were coated with gold and examined under SEM [58]. The elemental analysis was carried out with SEM equipped with Noran Six 200 Energy Dispersive X-ray (JEOL Ltd., Tokyo, Japan) [26].
3.6. Physicochemical Properties of a Partially-Purified Bioflocculant
3.6.1. MBF-UFH Concentration
Different concentrations (0.05, 0.1, 0.2, 0.3, 0.4 and 0.5 mg/mL) of purified MBF-UFH were made with distilled water, and the flocculating activity of each was determined by the flocculation assay [37].
3.6.2. Effect of Cations on Flocculating Activity
The synergistic effect of cations on the flocculating activity of purified MBF-UFH was investigated in accordance with the description of Zhao et al. [39]. The cation candidates included the metal of the following salts: NaCl, KCl, LiCl, MgCl2, CaCl2, MnCl2, FeCl3 and AlCl3. The flocculation assays were determined as described above, but CaCl2 was replaced by the solution of the aforementioned salts.
3.6.3. Effect of pH on the Flocculating Activity of MBF-UFH
The effect of pH on the flocculating activity was evaluated at a pH value ranging from 3–11; 4 g/L of the kaolin clay suspension was made and divided into 9 groups, and the pH of each group was adjusted accordingly with 0.1 M HCl and 0.1 M NaOH. The flocculating activity was determined while other conditions were kept constant [59].
3.6.4. Thermal Stability of MBF-UFH
MBF-UFH was dissolved in 60 mL of distilled water; 10 mL each of the bioflocculant solution were heated at different temperatures 50, 60, 70, 80, 90 and 100 °C for 1 h [50]. The bioflocculant solutions were allowed to cool, and their resulting flocculating activities were measured at room temperature, as described elsewhere [31].
3.7. Comparison of Flocculating Efficiencies of MBF-UFH and Conventional Flocculants
The flocculating efficiency of MBF-UFH and the conventionally-used chemical flocculants were compared by the flocculation assay in accordance with the description of Ugbenyen et al. [34] with some modifications. The chemical flocculants included: Alum, ferric chloride, anionic polyacrylamide (BDH chemicals Ltd.; Poole, UK, molecular weight ≥ 5000 KDa). Each of the flocculants was prepared by dissolving an appropriate concentration in distilled water, and jar tests were employed as described by Wang et al. [60]; the flocculating activities were measured separately afterwards.
3.8. Statistical Analysis
All data were treated in replicates, and the mean values were taken. Data were subjected to one-way analysis of variance (ANOVA) using the MINITAB Student Release 12 statistical package. A significance level of p ˂ 0.05 was used.
4. Conclusions
Bioflocculants are proposed to possess enormous industrial and biotechnological significance consequent to the advantages associated with them over conventionally-used flocculants. MBF-UFH produced by Bacillus sp. AEMREG7 under optimum fermentation conditions of 28 °C, 160 rpm and pH of six showed high flocculation activity of 83.2%. Bioflocculant production was, likewise, observed to be closely associated with cell growth. MBF-UFH’s composition was predominantly polysaccharides and proteins. The FTIR spectrum indicated the presence of hydroxyl and carboxyl in its molecular chain, which is important for flocculation. The SEM imaging illustrated the morphology and structural arrangements of MBF-UFH. As compared to conventionally-used flocculants, MBF-UFH demonstrated a high flocculating activity at a low dosage; a characteristic feature that demonstrates its suitability for industrial application. Thus, MBF-UFH potentially stands as an alternative candidate to chemical flocculants, hence making it attractive for further research and possible development for industrial-scale application.
Acknowledgments
We are grateful to the National Research Foundation (NRF) of South Africa, the Medical Research Council of South Africa and University of Fort Hare for funding this study.
Author Contributions
Anthony I. Okoh, Leonard V. Mabinya, Arinze S. Okoli and Uchechukwu U. Nwodo conceptualized the research; Kunle Okaiyeto collected data and prepared the first draft of the manuscript. All authors proofread the manuscript and approved the final draft for submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Jarvis P., Sharp E., Pidou M., Molinder R., Parson S., Jefferson B. Comparison of coagulation performance and floc properties using a novel zirconium coagulant against traditional ferric and alum coagulants. Water Res. 2012;46:4179–4187. doi: 10.1016/j.watres.2012.04.043. [DOI] [PubMed] [Google Scholar]
- 2.Hu C., Liu H.J., Qu J.H., Wang D.S., Ru J. Coagulation behavior of aluminium salts in eutrophic water: Significance of Al13 species and pH control. Environ. Sci. Technol. 2006;40:325–331. doi: 10.1021/es051423+. [DOI] [PubMed] [Google Scholar]
- 3.Shih I.L., van Y.T., Yeh L.C., Lin H.G., Chang Y.N. Production of a biopolymer flocculant from Bacillus licheniformis and its flocculation properties. Bioresour. Technol. 2001;78:267–272. doi: 10.1016/S0960-8524(01)00027-X. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Z., Wu C., Wu Y., Hu C. Comparison of coagulation performance and floc properties of a novel zirconium-glycine complex coagulant with traditional coagulants. Environ. Sci. Pollut. Res. 2014;21:6632–6639. doi: 10.1007/s11356-014-2575-7. [DOI] [PubMed] [Google Scholar]
- 5.Yang Q., Luo K., Liao D., Li X., Wang D., Liu X., Zeng G., Li X. A novel bioflocculant produced by Klebsiella sp. and its application to sludge dewatering. Water Environ. J. 2012;26:560–566. doi: 10.1111/j.1747-6593.2012.00319.x. [DOI] [Google Scholar]
- 6.Nwodo U.U., Green E., Mabinya L.V., Okaiyeto K., Rumbold K., Obi C.L., Okoh A.I. Bioflocculant production by a consortium of Streptomyces and Cellulomonas species and media optimization via surface response model. Coll. Surf. B Biointerfaces. 2014;116:257–264. doi: 10.1016/j.colsurfb.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Banks W.A., Niehoff M.L., Drago D., Zatta P. Aluminium complexing enhances amyloid protein penetration of blood–brain barrier. Brain Res. 2006;1116:215–221. doi: 10.1016/j.brainres.2006.07.112. [DOI] [PubMed] [Google Scholar]
- 8.Nwodo U.U., Agunbiade M.O., Green E., Mabinya L.V., Okoh A.I. A Freshwater Streptomyces, isolated from Tyume River, produces a predominantly extracellular glycoprotein bioflocculant. Int. J. Mol. Sci. 2012;13:8679–8695. doi: 10.3390/ijms13078679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y., Ye Z.-L., Fang X.-L., Li Y.-H., Cai W.-M. Production and characteristics of a bioflocculant produced by Bacillus sp. F19. Bioresour. Technol. 2008;99:7686–7691. doi: 10.1016/j.biortech.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 10.Liu W.J., Wang K., Li B.Z., Yuan H.L., Yang J.S. Production and characterization of an intracellular bioflocculant by Chryseobacterium daeguense W6 cultured in low nutrition medium. Bioresour. Technol. 2010;101:1044–1048. doi: 10.1016/j.biortech.2009.08.108. [DOI] [PubMed] [Google Scholar]
- 11.Mao Y.L., Tian C.X., Zhu J.W., Zhang T.Z., Tong L.B. Production of a novel biopolymer by culture of Bacillus cereus B-11 using molasses wastewater and its use for dye removal. Adv. Mater. Res. 2011;230:1119–1122. doi: 10.4028/www.scientific.net/AMR.230-232.1119. [DOI] [Google Scholar]
- 12.Abdel-Aziz S.M., Hamed H.A., Mouafi F.E., Abdelwahed N.A.M. Extracellular metabolites produced by a novel strain, Bacillus alvei NRC-14: 3. Synthesis of a bioflocculant that has chitosan-like structure. Life Sci. J. 2011;8:883–890. [Google Scholar]
- 13.Zhang Z., Xia S., Zhao J., Zhang J. Characterization and fiocculation mechanism of high efficiency microbial fiocculant TJ-F1 from Proteus mirabilis. Coll. Surf. B Biointerfaces. 2010;75:247–251. doi: 10.1016/j.colsurfb.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 14.He N., Li Y., Chen J., Lun S.-Y. Identification of a novel biofiocculant from a newly isolated Corynebacterium glutamicum. Biochem. Eng. J. 2002;11:137–148. doi: 10.1016/S1369-703X(02)00018-9. [DOI] [Google Scholar]
- 15.Yang Y.N., Ren N., Xue J.M., Yang J., Rong B.L. Mutation effect of MeV protons on biofiocculant bacteria Bacillus cereus. Nucl. Instrum. Methods Phys. Res. Sect. B Bean Interact. Mater. Atoms. 2007;262:222–224. [Google Scholar]
- 16.Li W.W., Zhou W.Z., Zhang Y.Z., Wang J., Zhu X.B. Flocculation behaviour and mechanism of an exopolysaccharide from the deep-sea psychrophilic bacterium Pseudoalteromonas sp. SM9913. Bioresour. Technol. 2008;99:6893–6899. doi: 10.1016/j.biortech.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 17.Mabrouk M.E.M. Production of bioflocculant by the marine actinomycete Nocardiopsis aegyptia sp. nov. Life Sci. J. 2014;11:27–35. [Google Scholar]
- 18.Nwodo U.U., Agunbiade M.O., Green E., Nwamadi M., Rumbold K., Okoh A.I. Characterization of an exopolymeric flocculant produced by a Brachybacterium sp. Materials. 2013;6:1237–1254. doi: 10.3390/ma6041237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manivasagan P., Venkatesan J., Sivakumar K., Kim S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014;169:262–278. doi: 10.1016/j.micres.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C., Kim S.K. Research and application of marine microbial enzymes: Status and prospects. Mar. Drugs. 2010;8:1920–1934. doi: 10.3390/md8061920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ugbenyen A., Cosa S., Mabinya L., Babalola O.O., Aghdasi F., Okoh A. Thermostable bacterial bioflocculant produced by Cobetia spp. isolated from Algoa bay (South Africa) Int. J. Environ. Res. Public Health. 2012;9:2108–2120. doi: 10.3390/ijerph9062108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J.-Y., Ye H.-F. Characterization and flocculating properties of an extracellular biopolymer produced from a Bacillus subtillis DYU1 isolate. Process Biochem. 2007;42:1114–1123. doi: 10.1016/j.procbio.2007.05.006. [DOI] [Google Scholar]
- 23.Mabinya L.V., Cosa S., Nwodo U., Okoh A.I. Studies on bioflocculant production by Arthrobacter sp. Raats, a freshwater bacteria isolated from Tyume River, South Africa. Int. J. Mol. Sci. 2012;13:1054–1065. doi: 10.3390/ijms13011054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajab Aljuboori A.H., Idris A., Abdullah N., Mohamad R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour. Technol. 2013;127:489–449. doi: 10.1016/j.biortech.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 25.He J., Zou J., Shao Z., Zhang J., Liu Z., Yu Z. Characteristics and flocculating mechanism of a novel bioflocculant HBF-3 produced by deep-sea bacterium mutant Halomonas sp. V3a’. World J. Microbiol. Biotechnol. 2010;26:1135–1141. doi: 10.1007/s11274-009-0281-2. [DOI] [Google Scholar]
- 26.Kavita K., Mishra A., Jha B. Extracellular polymeric substances from two biofilm forming Vibrio species: Characterization and applications. Carbohydr. Polym. 2013;94:882–888. doi: 10.1016/j.carbpol.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia S.S., Goyal D. Removal of heavy metal by waste tea leaves from aqueous solution. Eng. Life Sci. 2005;5:158–162. doi: 10.1002/elsc.200420066. [DOI] [Google Scholar]
- 28.Yin Y.-J., Tian Z.-M., Tang W., Li L., Song L.-Y., McElmurry S.P. Production and characterization of high efficiency bioflocculant isolated from Klebsiella sp. ZZ-3. Bioresour. Technol. 2014;171:336–342. doi: 10.1016/j.biortech.2014.08.094. [DOI] [PubMed] [Google Scholar]
- 29.Luo Z., Chen L., Chen C., Zhang W., Liu M., Han Y., Zhou J. Production and characteristics of a bioflocculant by Klebsiella pneumonia YZ-6 Isolated from human saliva. Appl. Biochem. Biotechnol. 2014;172:1282–1292. doi: 10.1007/s12010-013-0601-8. [DOI] [PubMed] [Google Scholar]
- 30.Freitas F., Alves V.D., Pais J., Costa N., Oliveira C., Mafra L., Hillious L., Oliveira R., Reis M.A.M. Characterization of an extracellular polysaccharide produced by a Pseudomonas strain grown on glycerol. Bioresour. Technol. 2009;100:859–865. doi: 10.1016/j.biortech.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Nie M., Yin X., Jia J., Wang Y., Liu S., Shen Q., Li P., Wang Z. Production of a novel bioflocculant MNXY1 by Klebsiella pneumoniae strain NY1 and application in precipitation of cyanobacteria and municipal wastewater treatment. J. Appl. Microbiol. 2011;111:547–558. doi: 10.1111/j.1365-2672.2011.05080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wan C., Zhao X.-Q., Guo S.-L., Alam M.A., Bai F.-W. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour. Technol. 2013;135:207–212. doi: 10.1016/j.biortech.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Wan Y.X., Wu L.P., Tang P.J., Zhang J.Y. Composition of extracellular polymeric substances produced by Geotrichum sp. J-5. Asian J. Chem. 2014;26:592–596. [Google Scholar]
- 34.Ugbenyen A.M., Okoh A.I. Characteristics of a bioflocculant produced by a consortium of Cobetia and Bacillus species and its application in the treatment of wastewaters. Water SA. 2014;40:139–144. doi: 10.4314/wsa.v40i1.17. [DOI] [Google Scholar]
- 35.Singh R.P., Shukla M.K., Mishra A., Kumari P., Reddy C.R.K., Jha B. Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr. Polym. 2011;84:1019–1026. doi: 10.1016/j.carbpol.2010.12.061. [DOI] [Google Scholar]
- 36.Salehizadeh H., Yan N. Recent advances in extracellular biopolymer flocculants. Biotechnol. Adv. 2014;32:1506–1522. doi: 10.1016/j.biotechadv.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Ma F., Qu Y., Sun D., Li A., Guo J., Yu B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium. radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol. 2011;27:2559–2565. doi: 10.1007/s11274-011-0726-2. [DOI] [Google Scholar]
- 38.Zaki S.A., Elkady M.F., Fareg S., Abd-EI-Haleem D. Characterization and flocculation properties of a carbohydrate bioflocculant from a newly isolated Bacillus velezensis 40B. J. Environ. Biol. 2013;34:51–58. [PubMed] [Google Scholar]
- 39.Zhao H.J., Liu H.T., Zhou J.G. Characterization of a biofiocculant MBF-5 by Klebsiella pneumoniae and its application in Acanthamoeba cysts removal. Bioresour. Technol. 2013;137:226–232. doi: 10.1016/j.biortech.2013.03.079. [DOI] [PubMed] [Google Scholar]
- 40.Yuan S.J., Sun M., Sheng G.P., Li Y., Li W.W., Yao R.S., Yu H.Q. Identification of key constituents and structure of the extracellular polymeric substances excreted by Bacillus megaterium TF10 for their flocculation capacity. Environ. Sci. Technol. 2011;45:1152–1157. doi: 10.1021/es1030905. [DOI] [PubMed] [Google Scholar]
- 41.Elkady M.F., Farag S., Zaki S., Abu-Elreesh G., Abd-El-Haleem D. Bacillus mojavensis strain 32A, a bioflocculant-producing bacterium isolated from an Egyptian salt production pond. Bioresour. Technol. 2011;102:8143–8151. doi: 10.1016/j.biortech.2011.05.090. [DOI] [PubMed] [Google Scholar]
- 42.Yim J.H., Kim S.J., Ahn S.H., Lee H.K. Characterization of a novel bioflocculant, p-KG03, from a marine dinoflagellate, Gyrodinium impudicum KG03. Bioresour. Technol. 2007;98:361–367. doi: 10.1016/j.biortech.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Okaiyeto K., Nwodo U.U., Mabinya L.V., Okoh A.I. Evaluation of the flocculation potential and characterization of bioflocculant produced by Micrococcus sp. Leo. Appl. Biochem. Microbiol. 2014;50:601–608. doi: 10.1134/S000368381406012X. [DOI] [Google Scholar]
- 44.Ugbenyen A.M., Cosa S., Mabinya L.V., Okoh A.I. Bioflocculant production by Bacillus sp. Gilbert isolated from a marine environment in South Africa. Appl. Biochem. Microbiol. 2014;50:49–54. doi: 10.1134/S0003683814010104. [DOI] [PubMed] [Google Scholar]
- 45.Gao Q., Zhu X.-H., Mu J., Zhang Y., Dong X.-W. Using Ruditapes philippinarum conglutination mud to produce bioflocculant and its applications in wastewater treatment. Bioresour. Technol. 2009;100:4996–5001. doi: 10.1016/j.biortech.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 46.Lian B., Chen Y., Zhao J., Teng H.H., Zhu L., Yuan S. Microbial fiocculation by Bacillus mucilaginosus: Applications and mechanisms. Bioresour. Technol. 2008;99:4825–4831. doi: 10.1016/j.biortech.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 47.Liu Z., Hu Z., Wang T., Chen Y., Zhang J., Yu J., Zhang T., Zhang Y., Li Y. Production of novel microbial flocculants by Klebsiella sp. TG-1 using waste residue from the food industry and its use in defecating the trona suspension. Bioresour. Technol. 2013;139:265–271. doi: 10.1016/j.biortech.2013.03.165. [DOI] [PubMed] [Google Scholar]
- 48.Salehizadeh H., Vossoughi M., Alemzadeh I. Some investigations on bioflocculant producing bacteria. Biochem. Eng. J. 2000;5:39–44. doi: 10.1016/S1369-703X(99)00066-2. [DOI] [Google Scholar]
- 49.Verma A.K., Dash R.R., Bhunia P. A review on chemical coagulation/fiocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012;93:154–168. doi: 10.1016/j.jenvman.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 50.Sun J., Zhang X., Miao X., Zhou J. Preparation and characteristics of bioflocculants from excess biological sludge. Bioresour. Technol. 2012;126:362–366. doi: 10.1016/j.biortech.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 51.Ntsaluba L., Nwodo U.U., Mabinya L.V., Okoh A.I. Studies on bioflocculant production by a mixed culture of Methylobacterium sp. Obi and Actinobacterium sp. Mayor. BMC Biotechnol. 2013;13:62. doi: 10.1186/1472-6750-13-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okaiyeto K., Nwodo U.U., Mabinya L.V., Okoh A.I. Characterization of a bioflocculant produced by a consortium of Halomonas sp. Okoh and Micrococcus sp. Leo. Int. J. Environ. Res. Public Health. 2013;10:5097–5110. doi: 10.3390/ijerph10105097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu C., Wang K., Jiang J.-H., Liu W.-J., Wang J.-Y. A novel biofiocculant produced by a salt-tolerant, alkaliphilic and biofilm-forming strain. Bacillus agaradhaerens C9 and its application in harvesting Chlorella minutissima UTEX2341. Biochem. Eng. J. 2015;93:166–172. [Google Scholar]
- 54.Cosa S., Ugbenyen A.M., Mabinya L.V., Rumbold K., Okoh A.I. Characterization and flocculation efficiency of a bioflocculant produced by a marine Halobacillus. Environ. Technol. 2013;34:2671–2679. doi: 10.1080/09593330.2013.786104. [DOI] [PubMed] [Google Scholar]
- 55.Li O., Lu C., Liu A., Zhu L., Wang P.-M., Qian C.-D., Jiang X.-H., Wu X.-C. Optimization and characterization of polysaccharide-based biofiocculant produced by Paenibacillus elgii B69 and its application in wastewater treatment. Bioresour. Technol. 2013;134:87–93. doi: 10.1016/j.biortech.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 56.Chaplin M.F., Kennedy J.F. Carbohydrate Analysis. 2nd ed. Oxford University Press; New York, NY, USA: 1994. [Google Scholar]
- 57.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 58.Jain R.M., Mody K., Joshi N., Mishra A., Jha B. Production and structural characterization of biosurfactant produced by an alkaliphilic bacterium, Klebsiella sp.: Evaluation of different carbon sources. Colloids Surf. B Biointerfaces. 2013;108:199–204. doi: 10.1016/j.colsurfb.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Wong Y.S., Ong S.-A., Teng T.-T., Aminah L.N., Kumaran K. Production of bioflocculant by Staphylococcus cohnii ssp. from Palm Oil Mill Effluent (POME) Water Air Soil Pollut. 2012;223:3775–3781. doi: 10.1007/s11270-012-1147-z. [DOI] [Google Scholar]
- 60.Wang Y., Gao B.Y., Yue Q.Y., Wei J.C., Zhou W.Z., Gu R. Color removal from textile industry wastewater using composite flocculants. Environ. Technol. 2010;28:629–637. doi: 10.1080/09593332808618824. [DOI] [PubMed] [Google Scholar]