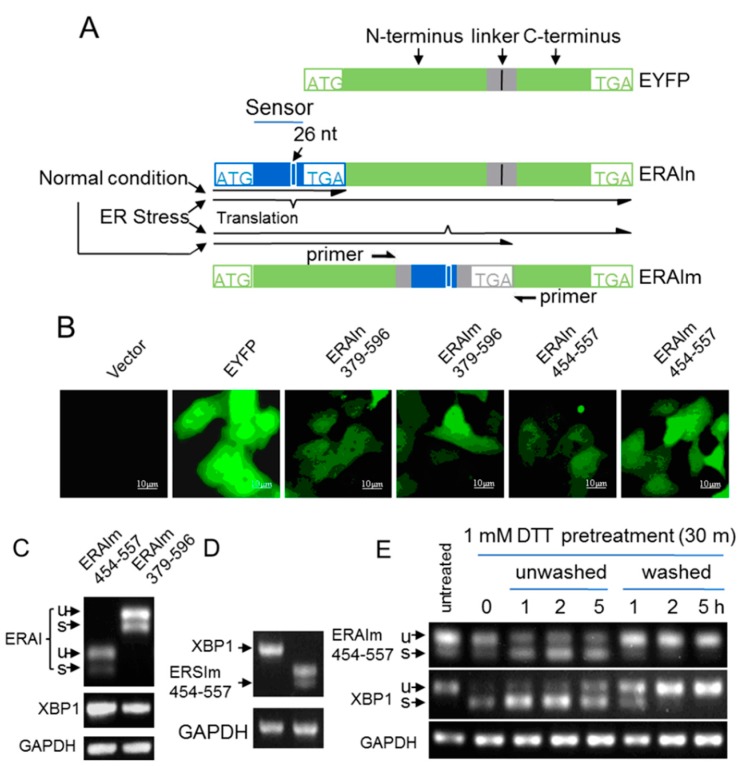
Figure 1.
Acute ER (endoplasmic reticulum) stress-independent splicing of XBP1 mRNA sensor sequence. (A) Schematic representation of ER stress activation indicators (ERAI). Two polypeptide linkers of RSIAT and RPACKIPNDGKQKVMNH were inserted between the 154A–155D residues of EYFP proteins, and they did not affect the fluorescence of EYFP. ER stress sensor sequences (as indicated by the numbers in (B)) of XBP1 mRNA were cloned into the 5′-end or between the linkers of EYFP (ERAIn and ERAIm). These ER stress sensor sequences of XBP1 mRNA contain the 26 nt intron, and only after the intron is removed, the shifted reading frames will express fluorescent proteins; (B) The fluorescence of MCF-7 cells expressing vector, EYFP, ERAIn379–596, ERAIm379–596, ERAIn454–557 and ERAIm454–557; (C) The total RNA of MCF-7/ERAIm454–557 and MCF-7/ERAI379–596 cells without treatment were subjected to RT-PCR with XBP1 primers and ERAI primers; (D) The total RNA of MCF-7/ERAIm454–557 cells was subjected to RT-PCT with XBP1 or ERAI primers and the PCR products were run in the agarose gel under the same condition. The GAPDH was the loading control; and (E) MCF-7/ERAIm454–557 cells were pretreated with 1 mM DTT (dl-Dithiothreitol) for 30 min (time 0), and then DTT was removed (washed) or continued to exist (unwashed). The total RNA was subjected to RT-PCR. u, unspliced; s, spliced.