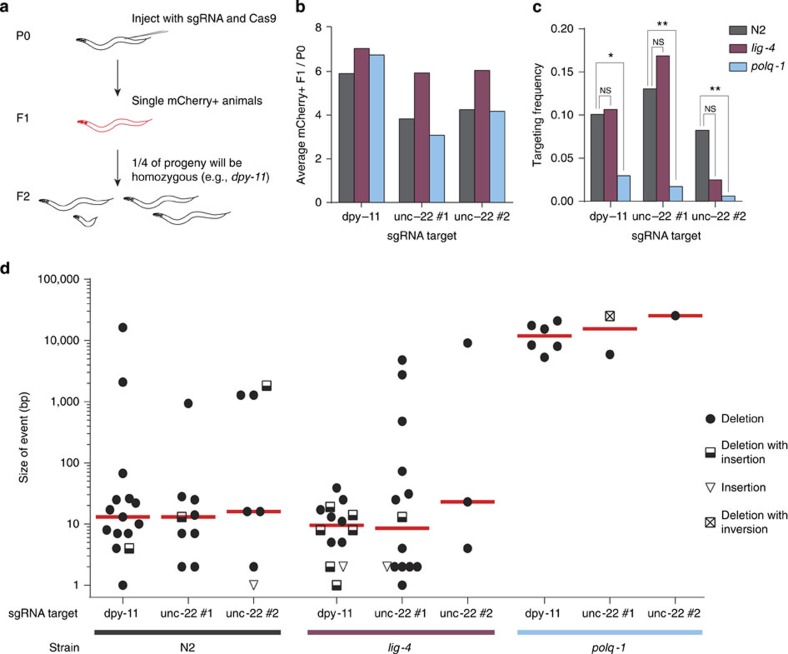
Figure 2. CRISPR/Cas9-induced mutations are generated through TMEJ.
(a) Schematic illustration of the strategy to generate mutants via CRISPR/Cas9 technology in C. elegans. Hermaphroditic animals (P0) are microinjected with plasmids that provide germline expression of Cas9 and of guide RNAs that target genes of interest (dpy-11 and unc-22). A marker plasmid that results in somatic mCherry expression was co-injected. Only mCherry-positive progeny animals (F1) were clonally grown because these have, when compared with non-expressing progeny animals, a higher chance of carrying a (heterozygous) mutation in the targeted gene. Homozygous mutant animals will manifest in a Mendelian manner in the brood (F2) of transformed F1's because of hermaphroditism. (b) A quantification of the efficiency of transgenesis in animals of different genotype. The average number of mCherry-expressing animals per injected P0 animal is indicated for each sgRNA target. More than 20 animals were injected per experimental condition. (c) A quantification of the efficiency of CRISPR/Cas9-induced gene targeting per sgRNA target in animals of different genotype. The frequency is defined as the number of mutant alleles divided by the number of successfully transformed F1 progeny animals. A Fisher's exact test was used to determine statistical significance. (NS, nonsignificant, *P<0.05, **P<0.01). (d) A size representation of CRISPR/Cas9-induced mutants that were obtained in wild-type, lig-4 and polq-1 mutant animals. Three different sgRNAs, targeting two genes were used. The median is indicated in red.