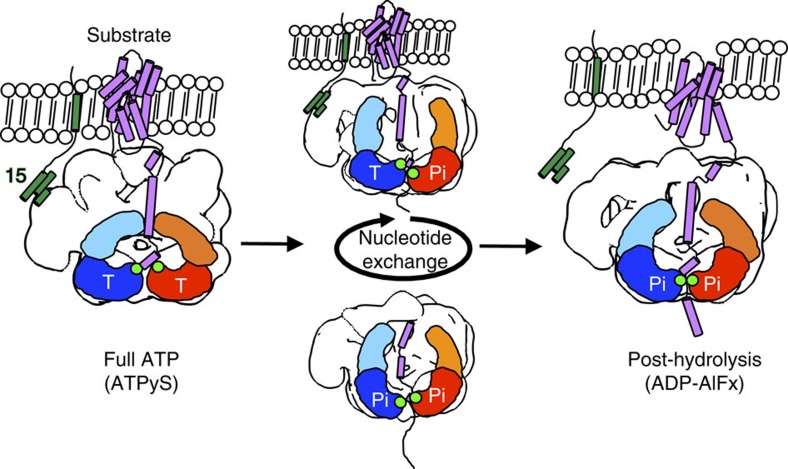
Figure 5. Model for Pex1/6 movements during ATP binding and hydrolysis.
The Pex1/6 complex anchors to the peroxisomal membrane via binding of Pex6 N domains to Pex15. Pex1 N domains establish interactions with the substrate. ATP binding to Pex1 D2 and Pex6 D2 (full ATP, ATPγS) elevates substrate-binding loops in the D2 domain, ready to grab the substrate. ATP turnover creates a power stroke that pulls the substrate along the central pore (post hydrolysis, ADP-AlFx). Nucleotide exchange in Pex6 D2 or Pex1 D2 translocates the substrate along the central pore (Pex1/6WBATP, Pex1WBATP/6). One Pex1 and Pex6 protomer are denoted as a simple cartoon representation. Conserved aromatic residues of substrate-binding loops are shown as green dots. Representative tertiary structures of substrate protein (purple) and membrane anchor Pex15 (green) are depicted as cartoon representations. Nucleotide occupancy of each D2 domain is indicated by T for ATP or Pi for the transition state.