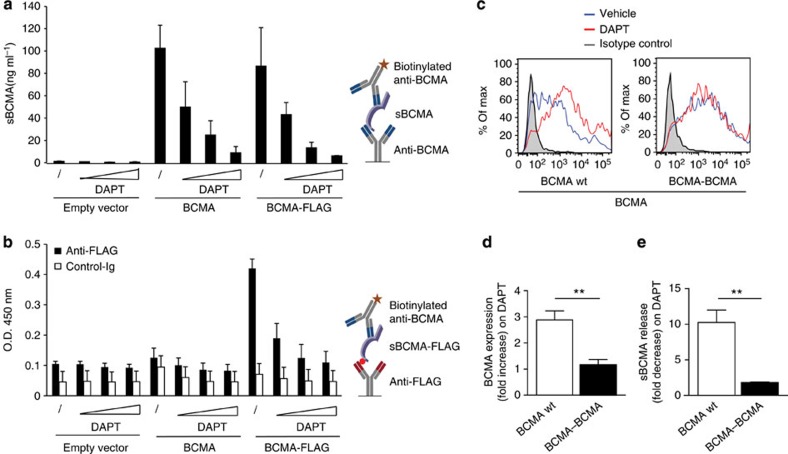
Figure 5. Release of sBCMA occurs without prior N-terminal trimming and is facilitated by the short extracellular domain of BCMA.
(a,b) HeLa cells were transfected with plasmids coding for full-length human BCMA or BCMA with an N-terminal FLAG and then cultured with increasing amounts of the γ-secretase inhibitor DAPT (0.02, 0.1 and 0.5 μM). Twenty-four hours after transfection supernatants were harvested and the released sBCMA was analysed using ELISA. In (a), ELISA wells were coated with anti-BCMA, and in (b) with anti-FLAG or a control IgG (anti-myelin oligodendrocyte glycoprotein (MOG) 8.18 C5), both were developed with anti-BCMA. Schemes of the ELISAs are shown on the right. Combined data of two independent experiments (mean±s.e.m.). (c–e) Human BCMA wild type (wt) or BCMA–BCMA with a doubled extracellular domain of BCMA were transfected into HEK293T cells. (c,d) Surface expression of BCMA was determined in the absence or presence of the γ-secretase inhibitor DAPT (1 μM). (d) The combined data of three experiments (P=0.0049, **P<0.01, unpaired t-test). (e) The effect of DAPT on the released sBCMA after transfection with BCMA wt or BCMA–BCMA (mean±s.e.m. of three experiments), P=0.0081, **P<0.01, unpaired t-test.