Abstract
Purpose: We evaluated the effects of α-blockers, antimuscarinics, or a combination of both in reducing ureteral stent-related symptoms.
Methods: The relevant studies were identified by searching MEDLINE, EMBASE and Cochrane Library Database from January 2000 to May 2014. Randomized controlled trials evaluating effects of α-blocker, antimuscarinic, and combination therapy for stent-related symptoms were included. Two reviewers independently screened studies and extracted data.
Results: A total of 13 articles were identified including 1408 patients. There were statistically significant differences in urinary symptom (−6.37; P<0.0001) and body pain index score (−7.03; P=0.0008) of the Ureteral Stent Symptom Questionnaire (USSQ), total International Prostate Symptom Score (IPSS) (−4.16; P=0.0006), Visual Analogue Pain Scale (VAPS) score (−2.48; P<0.00001), and quality of life (QoL) (−1.42; P=0.0009) in favor of the α-blocker group. Antimuscarinics alone vs the control group showed significant improvement in total IPSS (mean difference [MD]: −3.76; 95% confidence interval [CI], −5.08 to −2.43; P<0.00001) and QoL (MD: −0.82; 95% CI, −1.31 to −0.32; P=0.001). Compared with α-blockers monotherapy, combination therapy has significant lower total IPSS (MD: −3.74; 95% CI, −4.94 to −2.54; P<0.00001), VAPS (MD: −0.50; 95% CI, −0.89 to −0.11; P=0.01), and QoL (MD: −0.93; 95% CI, −1.30 to −0.55; P<0.00001).
Conclusions: Our data showed the beneficial effect of α-blockers alone and antimuscarinics alone in reducing stent-related symptoms. Furthermore, we suggested significant advantages of combination therapy of α-blocker and antimuscarinic compared with α-blocker monotherapy. However, more high quality, randomized controlled trials are warranted to better address this issue, however.
Introduction
Ureteral stents were first described in 1967, and placement has become a routine urologic procedure.1 The discomfort from placement of the stent, however, occurs significantly in some patients. Previous studies reported the incidence of frequency (50%–60%), urgency (57%–60%), dysuria (40%), incomplete emptying (76%), flank (19%–32%) and suprapubic pain (30%), and hematuria (25%).2–4 Joshi and associates5 demonstrated that stent-related urinary symptoms and pain result in reduced quality of life (QoL) in up to 80% of patients.5
The International Prostate Symptom Score (IPSS) is widely used for assessing lower urinary tract symptoms. It is not specific for stent-related symptoms, however. Then, a validated questionnaire called the Ureteral Stent Symptom Questionnaire (USSQ) was developed,6 which has been viewed as a reliable outcome measure for stent-related symptoms. Despite the growing evidence, the exact pathophysiology of stent-related symptoms is not clear.
Investigators have made considerable efforts to decrease stent-related symptoms, including improving stent materials, physical properties, and design.7 Unfortunately, the problem still exists. To solve this problem, several researchers investigated the effect of α-blockers on stent-related symptoms. Meanwhile, application of antimuscarinics or a combination of α-blockers and antimuscarinics for this indication was also proposed, but there remains controversy.
Recent meta-analyses were performed to confirm the beneficial effect of α-blockers alone in treatment of patients with ureteral stent symptoms, but the number of studies included in the articles is small.8,9 Therefore, we gathered the available prospective randomized controlled studies and performed a meta-analysis to evaluate the effects of α-blockers alone, antimuscarinics alone, and combination therapy in patients with an indwelling ureteral stent.
Methods
Literature search and study selection
We searched the databases MEDLINE, SCI, EMBASE, and The Cochrane Library from January 1, 2000, to January 2014. The key words of search strategy include “tamsulosin,” “alfuzosin,” “alpha blocker,” “antimuscarinic,” “stent,” “ureteral,” (and multiple synonyms for each term). Reference lists of the included studies were also searched. Two authors (LZ, XC) independently screened all citations and abstracts selected by the search strategy to identify potentially eligible studies. Abstracts presented but not published were included if useful information could be extracted. We tried to contact all corresponding authors when data were found to be missing.
Inclusion criteria and exclusion criteria
The study inclusion criterion was a randomized controlled trial (RCT) design of patients with ureteral stent insertion. Included studies compared treatment with α-blockers, antimuscarinics, or a combination against a control (placebo or no treatment). English language restriction was applied. Only studies on humans were included. Eligible trials that measured USSQ index, total IPSS, QoL score of IPSS, Visual Analogue Pain Scale (VAPS) were used for quantitative analysis. Data of at least one of these outcomes should be available in articles included in this review and could be combined. Disagreements on trial eligibility were resolved by consensus.
Data extraction and outcomes of interest
The primary outcome measures were urinary symptoms, pain, and QoL according to USSQ, IPSS, or VAPS. The USSQ is divided into six index areas: Urinary symptoms (11 questions), body pain (six questions), general health (six questions), work performance (seven questions), and sexual performance (four questions). Each question has a score giving a total score for each index. The IPSS was divided into the total score, obstructive symptom score, and storage symptom score. The VAPS is graded from 1 (minimal or no symptoms) to 10 (symptoms of maximal severity). Heterogeneity of outcomes was assessed to confirm the appropriateness of combining individual studies.
Study quality and level of evidence
The quality of studies was assessed using the Cochrane Collaboration criteria (Jadad scoring),10 including a judgment on randomization and quality of randomization, blinding and quality of allocation concealment, and description of dropouts.
Statistical analysis
Meta-analysis was performed with the Review manager 5.0 (The Cochrane Collaboration, Oxford, UK). P<0.05 was considered statistically significant. When a study did not provide the standard deviation (SD), it was calculated from a P value or imputed from other trials included the meta-analysis using the formula 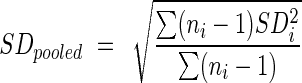 .9 The I2 was used to quantify statistical heterogeneity, with an I2>50% suggesting substantial statistical heterogeneity. Because studies used the same scale to measure outcomes, the mean difference (MD) of each score is calculated as the monotherapy or combination therapy score minus the control score.
.9 The I2 was used to quantify statistical heterogeneity, with an I2>50% suggesting substantial statistical heterogeneity. Because studies used the same scale to measure outcomes, the mean difference (MD) of each score is calculated as the monotherapy or combination therapy score minus the control score.
Results
Study characteristics
A total of 13 RCTs including 1408 patients were identified (Supplementary Fig. 1; supplementary data are available online at www.liebertpub.com/end). The characteristics of included trials are presented in Table 1. Seven RCTs enrolled patients undergoing insertion of a Double-J ureteral stent after ureteroscopic stone removal11–17; one RCT enrolled patients with unilateral ureteral stone-related hydronephrosis18 who had opted for conservative management with insertion of a Double-J ureteral stent; five RCTs enrolled patients with insertion of a Double-J ureteral stent after ureteral surgery for a variety of reasons, including ureteroscopy, percutaneous nephrolithotomy, and ureteroplasty, et al.19–23 All studies were published in English. The Jadad score varied two to five, and four studies had dropouts. Assessments of symptoms were completed in the range of 1 to 6 weeks.
Table 1.
Characteristics of Included Trials
| Author | Treatment | Outcomes | Duration | N | Control | Tα | TA | TC | Jadad |
|---|---|---|---|---|---|---|---|---|---|
| Beddingfieldl12 | Alfuzosin 10 mg | USSQ | 1 wk | 55 | 29 | 26 | 5 | ||
| Damiano11 | Tamsulosin 0.4 mg | USSQ | 1 wk | 75 | 37 | 38 | 3 | ||
| Deliveliotis18 | Alfuzosin 10 mg | USSQ | 1 wk | 100 | 50 | 50 | 5 | ||
| Dellis20 | Tamsulosin 0.4 mg/alfuzosin 10 mg | USSQ | 4 wk | 150 | 50 | 50/50 | 5 | ||
| Lee 13 | Tamsulosin 0.2 mg plus tolterodine 4 mg | IPSS, IPSSs, VAPS, QoL | 1 /4 wk | 53 | 18 | 15 | 20 | 2 | |
| Mo14 | Alfuzosin 10 mg | IPSS, VAPS, QoL | 1 wk | 29 | 16 | 13 | 5 | ||
| Navanimitkul21 | Tamsulosin 0.4 mg | IPSS, IPSSs, QoL | 1 wk | 42 | 21 | 21 | 3 | ||
| Nazim15 | Alfuzosin 10 mg | USSQ, VAPS | 2 wk | 130 | 65 | 65 | 5 | ||
| Park22 | Alfuzosin 10 mg | USSQ | 1 wk | 32 | 12 | 20 | 3 | ||
| Shalaby23 | Tamsulosin 0.4 mg plus solifenacin 10 mg | IPSS, IPSSs, VAPS, QoL | 6 wk | 327 | 81 | 82 | 80 | 84 | 3 |
| Tehranchi19 | Terazosin 4 mg plus tolterodine 2 mg | IPSS, IPSSs, QoL | 2 wk | 94 | 24 | 23 | 23 | 24 | 5 |
| Wang17 | Tamsulosin 0.4 mg | IPSS, IPSSs, VAPS, QoL | 2 wk | 146 | 71 | 75 | 4 | ||
| Wanga16 | Tamsulosin 0.4 mg | USSQ | 2 wk | 154 | 79 | 75 | 5 |
Two articles were included from Dr. Wang and were confirmed to be two independent trials.
Duration=duration of time after placement of the stent and treatment time; N=number of patients; Tα=α-blocker group; TA=antimuscarinic group; TC=combination group; USSQ=Ureteral Stent Symptom Questionnaire; IPSS=International Prostate Symptom Score; VAPS=Visual Analogue Pain Scale; QoL=quality of life; Jadad=the Cochrane Collaboration criteria (Jadad scoring).
α-blockers monotherapy
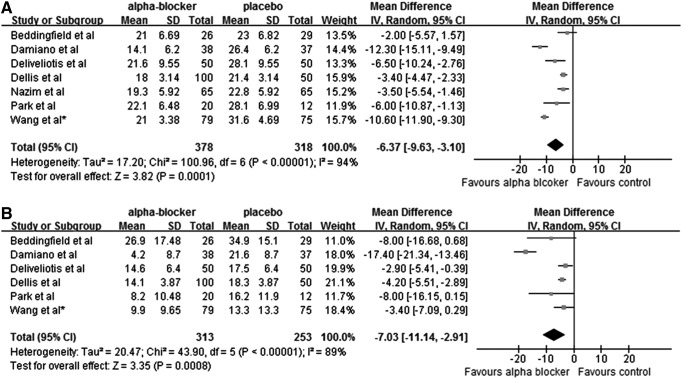
Seven randomized controlled studies including 696 patients have been published to assess the impact of an α-blocker on stent-related symptoms with USSQ. Alfuzosin was evaluated in four studies, tamsulosin in two studies, and both drugs in one study. Combining the results of those studies, α-blockers significantly decreased the urinary symptoms index score (−6.37 [−9.63 to −3.10]; P=0.0001) and pain index score (−7.30 [−11.14 to −2.91]; P<0.00001) when compared with control (Fig. 1, panels A and B). No further meta-analyses on other outcomes of USSQ were performed because of insufficient available data. We did not perform a test for funnel plot asymmetry because only seven studies were included in the meta-analysis.
Fig. 1.
Urinary symptoms (A) and pain (B) index score of the Ureteral Stent Symptom Questionnaire in the α-blocker group and control group. SD=standard deviation; CI=confidence interval.
Among the seven studies, some studies reported scores obtained at week 1 and week 4 whereas others reported only scores at week 1. To explore the impact of indwelling time of the stent before evaluating symptoms on activity of drugs, a subgroup analysis by drugs and indwelling time was performed. We found that no matter whether patients received alfuzosin or tamsulosin for 1 week or more than 4 weeks with the indwelling stent in situ, significant difference in urinary symptom index score was observed between the α-blocker group and the control group.
The mean reduction in the urinary symptom index score was 3.17 in the alfuzosin week 1 subgroup, 9.01 in the tamsulosin week 1 subgroup, 5.59 in the alfuzosin week 4 or week 6 subgroup, and 4.36 in the tamsulosin week 4 or week 6 subgroup when compared with control (Supplementary Fig. 2, panel A; supplementary data are available online at www.liebertpub.com/end). Not unexpectedly, the same trend was seen in the pain index score of four subgroups (Supplementary Fig. 2, panel B; supplementary data are available online at www.liebertpub.com/end).
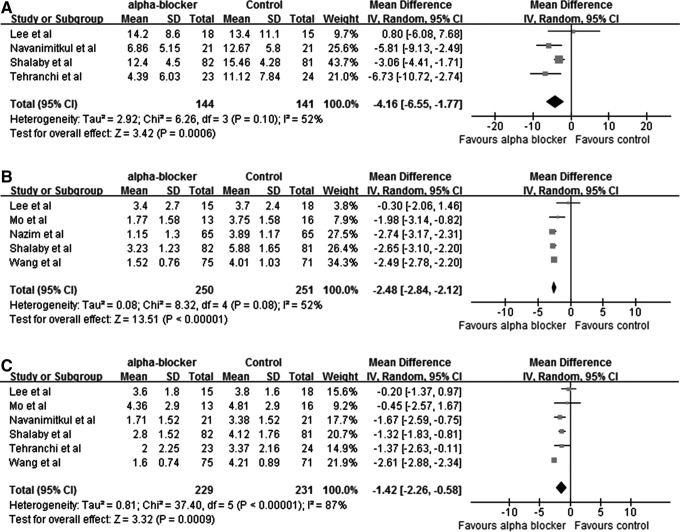
Although the IPSS was not validated, some studies adopted it to evaluate the stent-related symptoms. Six studies comparing the effect of α-blockers alone vs control with IPSS (five studies), VAPS (five studies), or QoL (six studies) of IPSS were also included and combined in the present meta-analysis. Among them, three studies evaluated tamsulosin and one evaluated terazosin. As shown in Figure 2, α-blockers are effective in improving the total IPSS (mean difference [MD]: −4.16; 95% confidence interval [CI], −6.55 to −1.77; P=0.0006), VAPS (MD: −2.48; 95% CI, −2.84 to −2.12; P<0.00001), and QoL of IPSS (MD: −1.42; 95% CI, −2.26 to −0.58; P=0.0009).
Fig. 2.
Total International Prostate Symptom Score (IPSS) (A), Visual Analogue Pain Scale (B), and quality of life of IPSS (C) in the α-blocker group and control group, respectively.
Antimuscarinics monotherapy
We included two studies on comparing antimuscarinics alone vs control for quantatitive analysis, which evaluated tolterodine and solifenacin, respectively. The results showed that antimuscarinics decreased the total IPSS (MD: −3.76; 95% CI, −5.08 to −2.43; P<0.00001). QoL (MD: −0.82; 95% CI, −1.31 to −0.32; P=0.001) was also improved (Fig. 3, panels A and B). VAPS was investigated in only one study (P<0.00001).
Fig. 3.
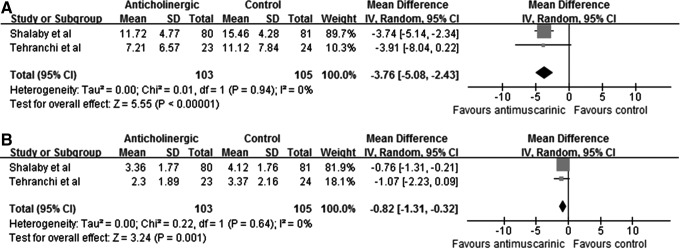
Total IPSS (A) and quality of life of IPSS (B) in the antimuscarinic group and control group, respectively.
Combination therapy
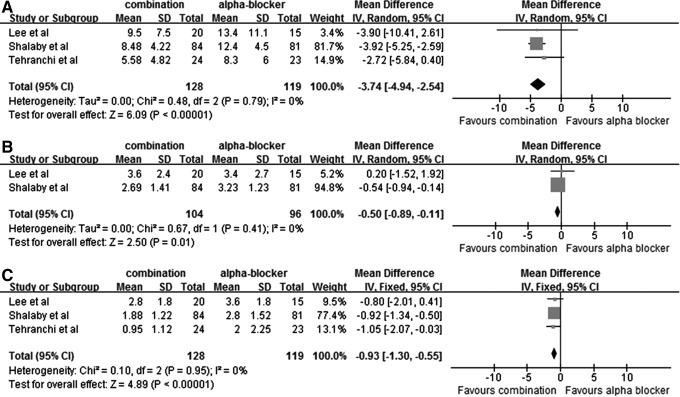
Three RCTS compared the combination therapy of α-blockers and antimuscarinics with α-blockers monotherapy. Patients who received combination therapy showed a statistically significant difference in total IPSS score (MD: −3.74; 95% CI, −4.94 to −2.54; P<0.00001), VAPS (MD: −0.50; 95% CI, −0.89 to −0.11; P=0.01) and QoL (MD: −0.93; 95% CI, −1.30 to −0.55; P<0.00001) (Fig. 4, panels A, B, C).
Fig. 4.
Total IPSS (A), Visual Analogue Pain Scale (B), and quality of life of IPSS (C) in the combination group and monotherapy group, respectively.
Safety
Because Lamb and colleagues8 and Yakoubi and coworkers9 summarized that adverse effects associated with α-blockers monotherapy were “minimal,” we only reported adverse effects related to antimuscarinics or combination therapy. Three studies indicated that no patients discontinued the treatments because of side effects. Shalaby and associates23 mentioned that therapies were well tolerated. In the study of Tehranchi and colleagues,19 3 patients in the antimuscarinic group had adverse effects (orthostatic hypotension and dry mouth) as did 11 patients in the combination therapy group (orthostatic hypotension, headache and dry mouth). The author found no significant differences among groups, however. Lee and coworkers13 reported that only one patient experienced dry mouth in combination therapy group.
Discussion
Because stent-related symptoms are similar to benign prostatic hyperplasia symptoms and overactive bladder symptoms, some studies reported that α-blockers or antimuscarinics may improve the symptoms. There is no overall review and systematic discussion in this field, however.
The sympathetic nervous system was reported to have a modulating role over ureteral peristalsis. Stimulation of α-receptors in the human ureters increases the force of ureteral contraction in response to the presence of a stone.24 Davenport and associates25 demonstrated that α-blockers may result in a significant reduction in the peak contraction pressure and lead to ureteral dilation. Flank pain may be from spasm of the ureter in patients with a stent. Therefore, α-blockers may decrease muscle spasm and reduced reflux of urine back to the kidney and may explain the ability to relieve flank pain. Meanwhile, improvement of urinary frequency and urgency may be because of blocking the α1 adrenoreceptors of the bladder trigone.
Consistent with previous reviews,8 our integrated analysis for USSQ using random effects demonstrated α-blockers have a beneficial role in relieving stent-related symptoms. We performed a subgroup analysis to show that both alfuzosin and tamsulosin showed significant effect. In most studies, the USSQ was not administered properly on the first and fourth weeks with the stent in situ, which was initially set by Joshi and colleagues.5 Previous meta-analyses did not take this factor into account either. It was supposed that longer duration of time after placement of the stent can improve overall tolerance.26 Therefore, we explored the impact of indwelling time before evaluating the effect of α-blockers by subgroup analysis. As shown in Supplementary Figure 2 (supplementary data are available online at www.liebertpub.com/end), α-blockers can still exert their clinical activity when patients received medical treatment for more than 4 weeks with the stent in situ.
Table 1 showed that IPSS was used as frequently as USSQ for assessing stent-related symptoms. Four RCTs using IPSS to measure outcomes of α-blockers were included to see if we can draw the same conclusion as that using the USSQ. Not unexpectedly, α-blockers could significantly decrease total IPSS, VAPS, and QoL in comparisons between the α-blocker and control groups. Even so, the USSQ is the only validated comprehensive questionnaire. It is developed by Joshi and coworkers3,5,6 for evaluating the symptoms and impact on health-related QoL because of ureteral stents. Patients with indwelling ureteral stents have a spectrum of symptoms, including frequency, urgency, dysuria, incomplete emptying, pain, and hematuria, et al. In this condition, none of the existing questionnaires is better than the USSQ in characterizing urinary symptoms associated with stents. Thus, we expected it to be a standard outcome measure for further studies on stent-related symptoms.
Mechanical stimulus coming from bladder coil and local trigone sensitivity could contribute to urinary frequency and urgency.3 Some researchers thought a ureteral stent may exacerbate preexisting subclinical detrusor overactivity and induce overactive bladder symptoms.3 In pathologic conditions, acetylcholine release could induce local contractions of the detrusor.27 Because muscarinic receptors mediated the involuntary bladder contraction caused by trigone irritation,19,22 antimuscarinics have been thought to block muscarinic receptors on the efferent in the detrusor muscle and reduce the ability of contraction and were considered first-line treatment for patients with overactive bladder.28 Oxybutynin was first reported to have no significant beneficial role in relieving stent-related symptoms.29 This is in contrast to that reported by others.13,22
In the present meta-analysis, two RCTs on antimuscarinics were shown to improve the total IPSS and QoL. Interestingly, whether antimuscarinics are effective in relieving stent-related pain remains a problem. Flank pain was associated with urine reflux from bladder to kidney, especially the voiding phase.30 Antimuscarinic agents at clinically recommended doses, however, have little effect on voiding contractions.
Combination therapy with a α-blocker and antimuscarinic has been well proven to be more effective than α-blocker monotherapy to improve overactive bladder symptoms.28,31 As for stent-related symptoms, Lim and colleagues32 reported in a nonrandomized, retrospective study that combined use of solifenacin and tamsulosin was significantly better than either drug alone.32 In contrast, Lee and coworkers13 in their prospective randomized study of more than 20 patients using a combination of tamsulosin and tolterodine reported no difference when compared with placebo and tamsulosin monotherapy. In this review, the pool analysis suggested the superiority of combination therapy in overcoming stent-related symptoms compared with α-blockers monotherapy.
Theoretically, antimuscarinic agents could inhibit detrusor muscle contraction and aggravate the voiding difficulties or cause urinary retention. On the basis of existing evidence from three studies, the incidence of side effects in the combination therapy group was not clinically more than that of α-blocker monotherapy, which is consistent with a previous study.31 More large-scale, prospective, and randomized studies on comparing combination therapy with monotherapy are needed.
This review contains several limitations. First, relevant original research in this area is limited, so we only assessed two studies with antimuscarinics and three studies with combination therapy. Second, analysis of heterogeneity with I2 presented high heterogeneity between α-blockers trials. Different drug therapy regimens, duration of stent, or reasons for stent insertion may be contributing factors, but we are still unable to explain the true reason for this. Third, adverse event descriptions were scarce, and we only offered the acquired data obtained from the literature.
Conclusion
In this meta-analysis, we showed that α-blockers alone were able to relieve urinary symptoms and pain that were caused by placement of stents. It also suggested that drug combination was statistically significantly better than α-blocker monotherapy and the effectiveness of antimuscarinics. There is need, however, for further studies to compare the effectiveness of a combination of α-blockers and antimuscarinics to optimize medical therapy for treatment of symptoms related to stent placement.
Supplementary Material
Abbreviations Used
- IPSS
International Prostate Symptom Score
- MD
mean difference
- QoL
quality of life
- RCT
randomized controlled trial
- SD
standard deviation
- USSQ
Ureteral Stent Symptom Questionnaire
- VAPS
Visual Analogue Pain Scale
Acknowledgments
This work was supported by the Ph.D. Programs Foundation of Ministry of Education of China (Priority area) (Grant No. 20110181130003), and the Science and Technology Bureau of Chengdu City (Grant No. 12PPYB030SF-002).
Disclosure Statement
No competing financial interests exist.
References
- 1.Zimskind PD, Fetter TR, Wilkerson JL. Clinical use of long-term indwelling silicone rubber ureteral splints inserted cystoscopically. J Urol 1967;97:840–844 [DOI] [PubMed] [Google Scholar]
- 2.Haleblian G, Kijvikai K, de la Rosette J, Preminger G. Ureteral stenting and urinary stone management: A systematic review. J Urol 2008;179:424–430 [DOI] [PubMed] [Google Scholar]
- 3.Joshi HB, Okeke A, Newns N, et al. . Characterization of urinary symptoms in patients with ureteral stents. Urology 2002;59:511–516 [DOI] [PubMed] [Google Scholar]
- 4.Thomas R. Indwelling ureteral stents: Impact of material and shape on patient comfort. J Endourol 1993;7:137–140 [DOI] [PubMed] [Google Scholar]
- 5.Joshi HB, Stainthorpe A, MacDonagh RP, et al. . Indwelling ureteral stents: Evaluation of symptoms, quality of life and utility. J Urol 2003;169:1065–1069 [DOI] [PubMed] [Google Scholar]
- 6.Joshi HB, Newns N, Stainthorpe A, et al. . Ureteral stent symptom questionnaire: Development and validation of a multidimensional quality of life measure. J Urol 2003;169:1060–1064 [DOI] [PubMed] [Google Scholar]
- 7.Dellis A, Joshi HB, Timoney AG, Keeley FX., Jr Relief of stent related symptoms: Review of engineering and pharmacological solutions. J Urol 2010;184:1267–1272 [DOI] [PubMed] [Google Scholar]
- 8.Lamb AD, Vowler SL, Johnston R, et al. . Meta-analysis showing the beneficial effect of alpha-blockers on ureteric stent discomfort. BJU Int 2011;108:1894–1902 [DOI] [PubMed] [Google Scholar]
- 9.Yakoubi R, Lemdani M, Monga M, et al. . Is there a role for alpha-blockers in ureteral stent related symptoms? A systematic review and meta-analysis. J Urol 2011;186:928–934 [DOI] [PubMed] [Google Scholar]
- 10.Jadad AR, Moore RA, Carroll D, et al. . Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control Clin trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 11.Damiano R, Autorino R, De Sio M, et al. . Effect of tamsulosin in preventing ureteral stent-related morbidity: A prospective study. J Endourol 2008;22:651–656 [DOI] [PubMed] [Google Scholar]
- 12.Beddingfield R, Pedro RN, Hinck B, et al. . Alfuzosin to relieve ureteral stent discomfort: A prospective, randomized, placebo controlled study. J Urol 2009;181:170–176 [DOI] [PubMed] [Google Scholar]
- 13.Lee SJ, Yoo C, Oh CY, et al. . Stent position is more important than alpha-blockers or anticholinergics for stent-related lower urinary tract symptoms after ureteroscopic ureterolithotomy: A prospective randomized study. Korean J Urol 2010;51:636–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mo K, Lee KS, Seo YJ, et al. . Effects of alpha-blocker on lower urinary tract symptoms due to ureteral stent: Prospective study. J Urol 2007;177:419–419 [Google Scholar]
- 15.Nazim SM, Ather MH. Alpha-blockers impact stent-related symptoms: A randomized, double-blind, placebo-controlled trial. J Endourol 2012;26:1237–1241 [DOI] [PubMed] [Google Scholar]
- 16.Wang CJ, Huang SW, Chang CH. Effects of specific alpha-1A/1D blocker on lower urinary tract symptoms due to double-J stent: A prospectively randomized study. Urol Res 2009;37:147–152 [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Huang SW, Chang CH. Effects of tamsulosin on lower urinary tract symptoms due to double-j stent: A prospective study. Urol Int 2009;83:66–69 [DOI] [PubMed] [Google Scholar]
- 18.Deliveliotis C, Chrisofos M, Gougousis E, et al. . Is there a role for alpha1-blockers in treating double-J stent-related symptoms? Urology 2006;67:35–39 [DOI] [PubMed] [Google Scholar]
- 19.Tehranchi A, Rezaei Y, Khalkhali H, Rezaei M. Effects of terazosin and tolterodine on ureteral stent related symptoms: A double-blind placebo-controlled randomized clinical trial. Int Braz J Urol 2013;39:832–840 [DOI] [PubMed] [Google Scholar]
- 20.Dellis AE, Keeley FX, Jr, Manolas V, Skolarikos AA. Role of alpha-blockers in the treatment of stent-related symptoms: A prospective randomized control study. Urology 2014;83:56–61 [DOI] [PubMed] [Google Scholar]
- 21.Navanimitkul N, Lojanapiwat B. Efficacy of tamsulosin 0.4 mg/day in relieving double-j stent-related symptoms: A randomized controlled study. J Int Med Res 2010;38:1436–1441 [DOI] [PubMed] [Google Scholar]
- 22.Park SC, Jung SW, Lee JW, Rim JS. The effects of tolterodine extended release and alfuzosin for the treatment of double-j stent-related symptoms. J Endourol 2009;23:1913–1917 [DOI] [PubMed] [Google Scholar]
- 23.Shalaby E, Ahmed AF, Maarouf A, et al. . Randomized controlled trial to compare the safety and efficacy of tamsulosin, solifenacin, and combination of both in treatment of double-j stent-related lower urinary symptoms. Adv Urol 2013;2013:752382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davenport K, Timoney AG, Keeley FX. A comparative in vitro study to determine the beneficial effect of calcium-channel and alpha(1)-adrenoceptor antagonism on human ureteric activity. BJU Int 2006;98:651–655 [DOI] [PubMed] [Google Scholar]
- 25.Davenport K, Timoney AG, Keeley FX., Jr Effect of smooth muscle relaxant drugs on proximal human ureteric activity in vivo: A pilot study. Urol Res 2007;35: 207–213 [DOI] [PubMed] [Google Scholar]
- 26.Irani J, Siquier J, Pires C, et al. . Symptom characteristics and the development of tolerance with time in patients with indwelling double-pigtail ureteric stents. BJU Int 1999;84:276–279 [DOI] [PubMed] [Google Scholar]
- 27.Andersson KE. Antimuscarinics for treatment of overactive bladder. Lancet Neurol 2004;3:46–53 [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi O. Latest treatment for lower urinary tract dysfunction: Therapeutic agents and mechanism of action. Int J Urol 2013;20:28–39 [DOI] [PubMed] [Google Scholar]
- 29.Norris RD, Sur RL, Springhart WP, et al. . A prospective, randomized, double-blinded placebo-controlled comparison of extended release oxybutynin versus phenazopyridine for the management of postoperative ureteral stent discomfort. Urology 2008;71:792–795 [DOI] [PubMed] [Google Scholar]
- 30.Mosli HA, Farsi HM, al-Zimaity MF, et al. . Vesicoureteral reflux in patients with double pigtail stents. J Urol 1991;146:966–969 [DOI] [PubMed] [Google Scholar]
- 31.Hao N, Tian Y, Liu W, et al. . Antimuscarinics and alpha-blockers or alpha-blockers monotherapy on lower urinary tract symptoms—A meta-analysis. Urology 2014;83:556–562 [DOI] [PubMed] [Google Scholar]
- 32.Lim KT, Kim YT, Lee TY, Park SY. Effects of tamsulosin, solifenacin, and combination therapy for the treatment of ureteral stent related discomforts. Korean J Urol 2011;52:485–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.