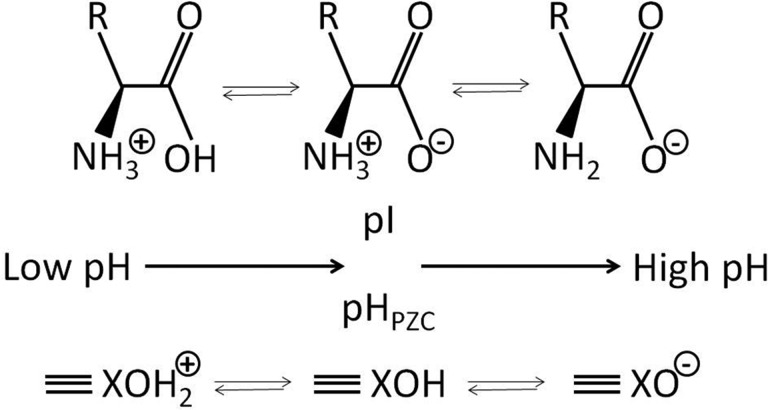
FIG. 1.
General molecular structure of L-α-amino acids, revealing their zwitterionic characteristics and the dependence of the charge of their carboxylic acid and α-amino groups on pH. At a pH equal to the isoelectric point (pI), the net charge is zero. R is the side chain specific to each amino acid. The bottom part shows a hydroxide mineral, which reveals a similar impact of pH on charge, with pHpzc indicating the pH corresponding to the point of zero charge (pzc).