Table 1.
Standard Molal Thermodynamic Data (25°C and 1 bar) and the Revised Helgeson–Kirkham–Flowers Equation States for Aqueous Species
| Species | ΔfGo,a | ΔfHo,a | 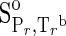 |
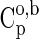 |
Vo,c | a1×10d | a2×10−2,e | 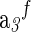 |
a4×10−4,g | 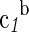 |
c2×10−4,g | ω×10−5,e |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glyh | −88.62 | −122.83 | 37.89 | 9.3 | 43.2 | 11.30 | 0.71 | 3.99 | −3.04 | 28.5 | −8.40 | 0.23 |
| Alai | −88.81 | −132.50 | 38.83 | 33.6 | 60.4 | 14.90 | 1.74 | 7.16 | −3.69 | 49.5 | −7.00 | 0.18 |
| Leui | −84.20 | −153.60 | 50.41 | 95.2 | 107.8 | 24.68 | 7.51 | 19.93 | −8.37 | 102.7 | −3.30 | 0.09 |
| GlyGlyh | −117.00 | −175.69 | 52.75 | 28.0 | 77.4 | 17.66 | 6.71 | 50.07 | −15.91 | 69.3 | −17.71 | 0.59 |
| AlaGlyj | −116.73 | −186.11 | 50.70 | 60.3 | 95.2 | 14.65 | 21.16 | 12.08 | −3.65 | 54.7 | 0.80 | −0.43 |
| LeuGlyj | −110.62 | −202.66 | 72.60 | 118.8 | 145.3 | 21.40 | 34.65 | 13.18 | −4.21 | 98.6 | 6.48 | −0.76 |
| GlyGlyGlyh | −144.74 | −226.97 | 70.73 | 44.2 | 112.1 | 24.14 | 6.97 | 33.69 | −10.69 | 63.9 | −16.60 | −1.54 |
| Adeninek | 74.77 | 31.24 | 53.41 | 56.2 | 89.6 | 21.50 | 8.50 | −2.66 | −5.36 | 87.9 | −15.87 | 0.07 |
| Ribosek | −179.74 | −247.13 | 59.53 | 66.5 | 95.7 | 22.65 | 7.29 | −5.39 | −3.41 | 134.7 | −32.82 | 0.17 |
| Adenosinek | −46.50 | −148.49 | 87.19 | 120.3 | 170.7 | 39.55 | 12.90 | 8.97 | −8.82 | 163.2 | −20.10 | 0.23 |
