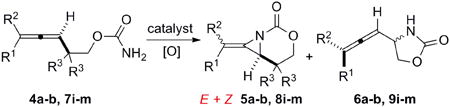
Table 3.
Chemoselective aziridination of homoallenic carbamates using Ag(I) catalysis.
| |||||
|---|---|---|---|---|---|
|
| |||||
| entry | desired product | Catalysta,b | E:Z | A:lc | yield |
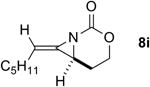
| 1 |
|
Rh2TPA4 | 4:1 | 4:1 | 80% |
| Rh2(esp)2 | 3:1 | 4:1 | 66% | ||
| AgOTf/bipy | 3:1 | 4:1 | 72% | ||
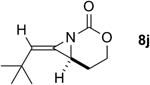
| 2 |
|
Rh2(esp)2 | >99:1 | 2:1 | 49% |
| AgOTf/phen | >99:1 | 1:1 | 40%d | ||
| AgOTf/bipy | >99:1 | 3.7:1 | 67% | ||
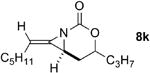
| 3 |
|
Rh2(esp)2 | --- | 1:1 | 34% |
| AgOTf/phen | 4.8:1 | 9:1 | 80%e | ||
| 4 |
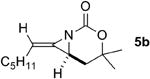
|
Rh2(esp)2 | 2:1 | 1:17 | 5% |
| AgOTf/phen | 1.9:1 | 5.9:1 | 70% | ||
| 5 |
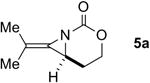
|
Rh2(esp)2 | --- | 2:1 | 35% |
| AgOTf/phen | --- | >20:1 | 79% | ||
| 6 |
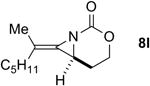
|
Rh2(esp)2 | 2.3:1 | 1:1.3 | 34% |
| AgOTf/phen | 2.6:1 | 11.5:1 | 87% | ||
| 7 |
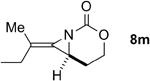
|
Rh2(esp)2 | --- | 1.1:1 | 32% |
| AgOTf/phen | 2.3:1 | 19:1 | 70% | ||
Rh conditions: 3 mol % catalyst, 2 equiv PhIO, 4 Å MS, CH2CI2, rt.
Reaction conditions: 20 mol % AgOTf, 25 mol % ligand, 2 equiv PhIO, 4 Å MS, CH2CI2, rt.
A = aziridination; I = insertion.
73% conversion.
dr E = 1:1; dr Z = 3:1.
