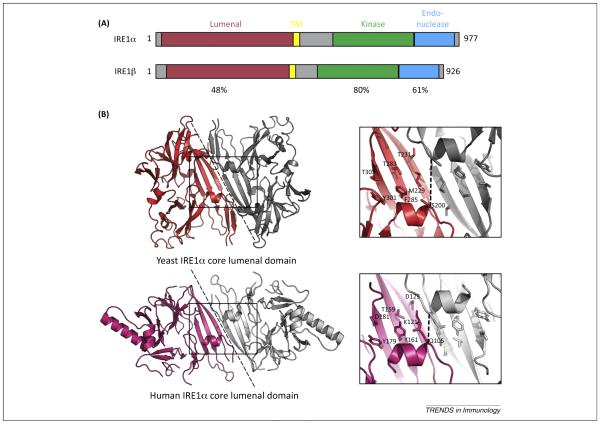
Figure 1.
IRE1 domain organization and structure. (A) Schematic shows domain structures of human IRE1α and IRE1β with sequence homologies indicated. (B) Crystal structures of lumenal domain from (top) yeast IRE1 (PDB 2BEI, [17]) and (bottom) human IRE1α (PDB 2BZ6, [18]). The dotted line represents the proposed dimer interfaces observed in the crystal structures. The boxed region is enlarged in the right panels to illustrate the proposed MHC-like peptide-binding grooves. Key residues in the binding groove are indicated for one protomer of the dimer. The width of the groove in yeast IRE1 (as measured between Cα atoms of Ser 200 in each protomer) is 10.7 Å. The width of the groove in human IRE1α is reduced (6.8 Å between Cα atoms of Gln 105) and is occluded by the side chain of Gln 105.