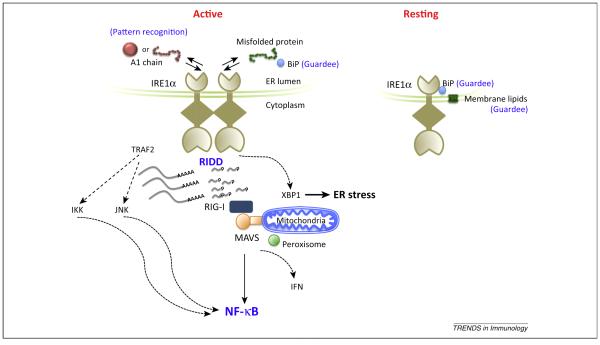
Figure 2.
The IRE1–RIDD–RIG-I pathway. The IRE1–RIDD–RIG-I pathway connects the ER with innate immune signaling in response to some forms of the microbial environment and other forms of cellular distress affecting the ER. In the resting state, IRE1α occupies the ER membrane as a monomer bound to Bip. The lumenal domain monitors the ER for dysfunction or signs of danger, which are marked at least in part by the loss of Bip binding (a putative guardee). The transmembrane domain monitors the ER membrane for perturbations in lipid structure (a second putative guardee). Activation likely also depends on IRE1α binding to signs of danger – either misfolded endogenous proteins or invading microbial products. In the case of cholera toxin, very small amounts of the A1-chain entering the ER appear to bind IRE1α, although we do not yet know in what conformation (folded or unfolded). The reaction suggests a role for IRE1α as a pattern recognition receptor. On activation IRE1α oligomerizes and the cytosolic kinase and endonuclease domains are activated to splice XBP-1 mRNA for translation into the active transcription factor, and to cleave other mRNA into fragments that lack markers of self, thus rendering them ligands for the antiviral sensor RIG-I. This general endonuclease activity is termed RIDD. Activation of RIG-I via MAVS leads to an inflammatory response. IRE1α can also induce an inflammatory response by scaffolding to TRAF2 and activating JNK- or IKK-dependent pathways.