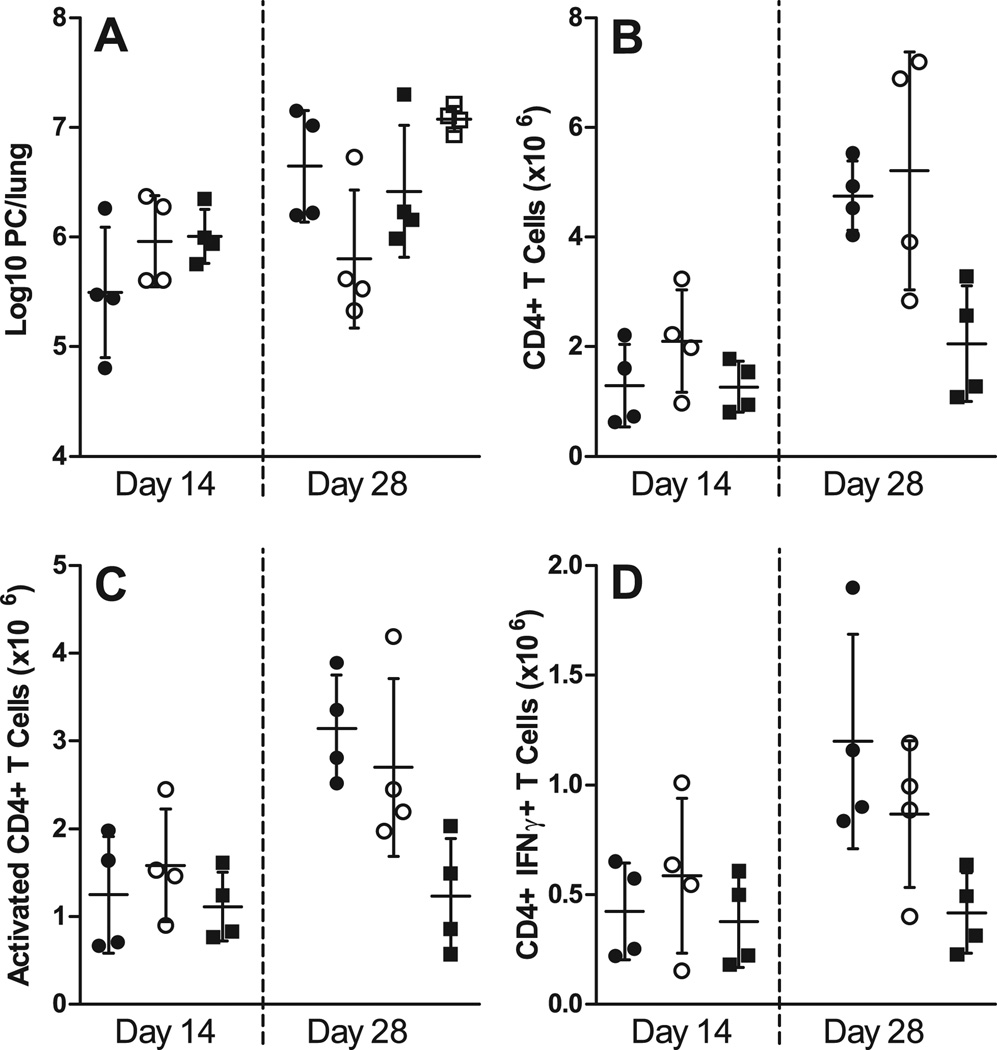
FIGURE 7.
Ag-specific BCR is required for proper CD4+ T cell priming. WT, µMT, and MD4 chimeras were infected with PC, and after 3 days T cells were adoptively transferred into Rag1−/− mice, which were then infected with PC 4 days later. Mice receiving T cells from µMT chimeras (closed circles), WT (B6+µMT) chimeras (open circles), MD4 chimeras (closed squares), and mice that were unreconstituted (open squares) were then humanely killed at post-infection timepoints. (A) PC lung burden was assessed in lung digest samples and expressed as Log10 PC organisms per lung on days 14 and 28 post-infection. The numbers of (B) CD4+ T cells, (C) activated CD4+ T cells (based on high CD44 and low CD62L expression), and (D) CD4+ T cells producing IFNγ (assessed via intracellular cytokine staining and flow cytometry) are depicted at the corresponding timepoints. Data are presented as mean ± SD along with symbols representing each individual mouse. No statistically significant differences were observed defined at a p-value of < 0.05 using ANOVA and Dunn’s post hoc test for data compared to the WT chimera (B6+µMT) group at each timepoint.