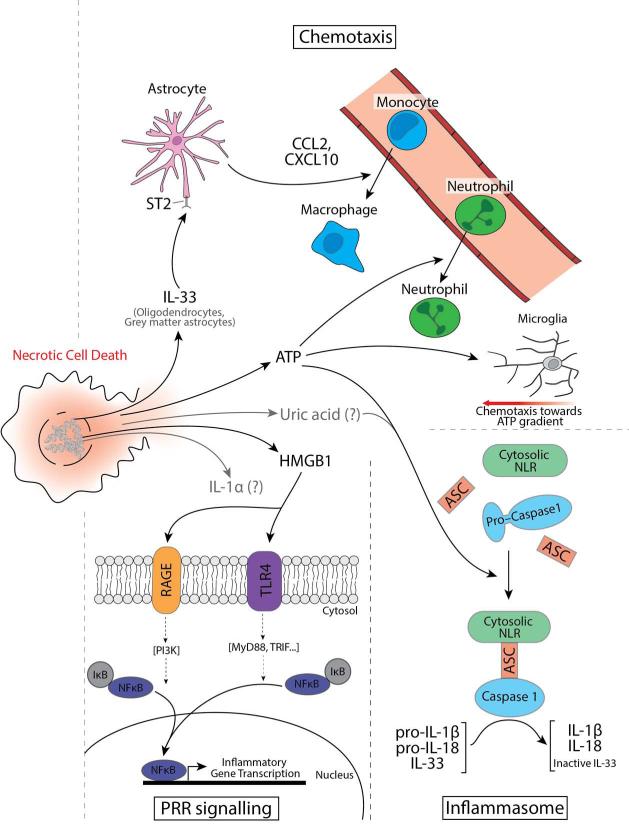
Figure 2. Necrotic cell death causes the release alarmins into the extracellular space.
Necrotic cell death releases peptide and nucleic acid derivative alarmins that initiate inflammation. IL-33 plays an important role in bringing monocyte-derived macrophages into the CNS through upregulation of astrocytic chemokine expression. ATP promotes chemotaxis of neutrophils (through its activation of the inflammasome), and is directly chemotactic to microglial processes. ATP and uric acid also activates the inflammasome, stimulating the assembly of the cytosolic NLR, ASC, and pro-caspase 1. Pro-caspase 1 is auto-cleaved to mature caspase 1, which cleaves pro-IL-1β and pro-IL-18 to active forms and IL-33 to an inactive form. HMGB1 acts as on TLR4 and RAGE receptors and directly promotes inflammatory cytokine and chemokine production. An important transcription factor downstream of both receptors is NF-κB, important in enhancing inflammation and cellular infiltration, but the RAGE receptor has several other downstream signaling pathways (not shown here). IL-1α and uric acid (gray arrows), are important inducers of immune responses in response to tissue damage in the periphery, however their roles in response to CNS injury remained poorly defined.