Abstract
Objective: The purpose of this study was to investigate whether the presence of tic disorder is negatively associated with sertraline (SRT) outcomes, but not with continued cognitive-behavioral therapy (CBT), in a sample of youth who were unresponsive to an initial full course of CBT.
Methods: In the Nordic Long-Term OCD Study, children and adolescents with OCD who were rated as nonresponders to 14 weeks of open-label CBT were randomized to continued CBT (n=28) or SRT treatment (n=22) for an additional 16 weeks of treatment. We investigated whether the presence or absence of comorbid tic disorder moderated treatment outcomes on the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS).
Results: Twelve out of 50 (24.0%) participants were diagnosed with comorbid tic disorder, with 7 receiving continued CBT and 5 receiving SRT, respectively. In patients without tic disorder, results showed no significant between-group differences on average CY-BOCS scores. However, in patients with comorbid tic disorder, those who received SRT had significantly lower average CY-BOCS scores than those who received continued CBT.
Conclusions: Children and adolescents with OCD and comorbid tic disorder, who are nonresponders to an initial 14 week course of CBT, may benefit more from a serotonin reuptake inhibitor (SRI) than from continued CBT.
Introduction
Pediatric obsessive-compulsive disorder (OCD) is a chronic and disabling disorder with a prevalence rate between 1% and 2% (Rapoport et al. 2000; Ruscio et al. 2010). Cognitive-behavior therapy (CBT) and selective serotonin reuptake inhibitors (SSRI) have been shown to be effective treatments, and are recommended as first-line interventions for this population (American Academy of Child and Adolescent Psychiatry 2012). However, non- or only partial response to these first-line treatments is common (Pediatric OCD Treatment Study [POTS] Team 2004; Abramowitz et al. 2005; Watson and Rees 2008; Skarphedinsson et al. 2015, Ivarsson et al., in press). Unfortunately, we are far from understanding the causes of partial response. Several factors influencing treatment response have been implicated, such as initial severity of OCD symptoms and comorbid psychiatric disorders (Ginsburg et al. 2008; Garcia et al. 2010; Torp et al., 2015a).
Comorbidity between OCD and tic disorders is high within pediatric populations, with 20–59% of children and adolescents with OCD also meeting criteria for a tic disorder (Riddle et al. 1990; Leonard et al. 1992; Toro et al. 1992; Zohar et al. 1992; Hanna 1995; Thomsen and Mikkelsen 1995; Ivarsson et al. 2008). Tic disorders are also more prevalent in those with childhood-onset OCD than in those with adult-onset OCD. This finding has led some researchers to speculate that childhood-onset OCD represents a phenomenologically and etiologically distinct type of OCD that may bear a close genetic relationship to tic disorders, perhaps sharing the same or similar pathogenesis (Pauls et al. 1995; Eichstedt and Arnold 2001; Peterson et al. 2001). Furthermore, the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) specifies tic-related OCD in patients with OCD and a current or past history of a tic disorder (American Psychiatric Association 2013).
Previous researchers have studied tic disorders, both as a predictor and moderator of OCD treatment outcomes. Overall, results have been inconsistent. In adults, two uncontrolled SSRI trials exist. One study showed poorer outcomes for patients with comorbid tic disorders (McDougle et al. 1993), whereas the other showed no difference (Husted et al. 2007). There exist at least eight studies examining tics as either a predictor or moderator of treatment outcomes in OCD in pediatric populations. Three uncontrolled studies of CBT (Piacentini et al. 2002; Himle et al. 2003; Torp et al., 2015a) and one RCT combining intensive and weekly CBT (Storch et al. 2008) did not find that the presence of tic disorder predicted treatment outcome. One uncontrolled trial of SSRIs reported poorer acute outcomes (Geller et al. 2003) for patients with comorbid tic disorders. These findings were replicated in a more methodologically rigorous study (March et al. 2007). In this study, patients with comorbid tic disorder showed poorer outcome when randomized to sertraline (SRT) alone when compared with CBT or the combination of CBT and SRT. However, in another RCT evaluating SRT versus placebo, the acute outcome was no worse for patients with comorbid tic disorder (March et al. 1998). Likewise, in a recent study of SSRI partial responders randomized to continued SSRI, SSRI and instructions in CBT, or SSRI and full CBT, participants with and without comorbid tics fared equally well across treatments (Conelea et al. 2014).
Data to date suggest partially that the presence of a tic disorder in youth with OCD might predict poorer outcomes, but only when children and adolescents are treated with an SSRI. However, there are several challenges to this conclusion. First, the number of studies examining this question is small, with only three randomized controlled trials. Second, the operational definition of tic comorbidity varies widely across studies. In one study, participants were excluded who met diagnostic criteria for Tourette's disorder, so the analysis was performed by grouping patients with a chronic motor or vocal tic disorder (Geller et al. 2003). One study defined “tics” as the presence of any tic symptom, in order to increase the number of patients with tics in their analysis (March et al. 2007) and another used the recent DSM-5 definition of current or past history of tic disorder (Conelea et al. 2014). Third, the treatments evaluated varied significantly. Three of the four CBT trials included patients on medications (Piacentini et al. 2002; Himle et al. 2003; Storch et al. 2008), limiting the conclusions that can be reached with respect to CBT. Two SRI trials did not report anything about concurrent treatment (March, et al. 1998; Geller et al. 2003), whereas two of the RCTs excluded patients who had failed a prior course of CBT (March et al. 2007; Conelea et al. 2014) or who had failed two adequate trials of an SSRI (March et al. 2007). It is challenging to draw firm conclusions from the current available literature, as treatment-naïve and treatment-resistant patients were combined. Therefore, the question of tic disorders serving as a clinically important predictor or moderator of treatment outcomes in pediatric OCD is still open for empirical scrutiny.
Adult OCD studies indicate that patients with OCD and tic disorder, when compared with those without a comorbid tic disorder, have more severe OCD and comorbid mood, anxiety, attention-deficit/hyperactivity disorder (ADHD), and substance use disorder (Coffey et al. 1998; Cath et al. 2001). Likewise, Lewin and colleagues (2010) reported evidence that the pattern of comorbidity is different in OCD patients with and those without tics. They showed that baseline OCD severity did not differ between participants with OCD and those with OCD and comorbid tic disorders. However, anxiety disorders were more frequent among those OCD participants without a comorbid tic disorder, whereas ADHD and oppositional defiant disorder (ODD)/conduct disorder (CD) was more frequent in those OCD participants with a comorbid tic disorder. It is not entirely clear whether internalizing or externalizing disorders/symptoms are predictors or moderators of OCD treatment outcome, as studies to date have also been inconsistent with respect to this question. However, the majority of studies suggest that internalizing symptoms/disorders are not predictive of treatment outcome, although externalizing disorders do predict poorer outcomes (Ginsburg et al. 2008; Garcia et al. 2010; Torp et al., 2015a). It is likely that skewed representation of comorbid internalizing and externalizing disorders might influence the association of OCD-specific treatment outcome and comorbid tic disorder.
In the Nordic Long-Term OCD Treatment Study (NordLOTS), 269 children and adolescents with OCD were treated with 14 weekly CBT sessions. Clinical response was defined as a Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) total score of ≤15 at posttreatment, assessed by independent evaluators (IEs). Based on this criterion, 27.4% (n=66) of the original 269 patients were determined to be nonresponders (Torp et al. 2015). These patients were then eligible to be randomized to either SRT (n=22) or continued CBT (n=28). Twelve of these 50 patients had comorbid tic disorder.
Based on previous literature, we had the following hypotheses: 1) Presence of tic disorder will be associated with worse outcomes among those randomly assigned to receive SRT, but not among those assigned to continued CBT; 2) externalizing disorders will be overrepresented in the tic group, which will influence the association between outcome and the presence of tic disorder; and 3) internalizing disorders, although overrepresented in the OCD group without tics, will not influence the association between outcome and comorbid tic disorder.
Methods
Design
NordLOTS is a multinational, stepped-care study comparing treatment effects of CBT and SRT among children who received 14 weeks of individual CBT in a first step (Step 1) and were classified as treatment nonresponders (Ivarsson et al. 2010; Skarphedinsson et al. 2013; Thomsen et al. 2013; Torp, et al. 2015b). The trial was approved by the Norwegian, Swedish, and Danish Committees for Medical and Health Research Ethics and the Medical Products Agencies. The project was registered in Current Controlled Trials (www.controlled-trials.com ISRCTN66385119). Informed consent was provided by parent(s) or guardian(s), and informed assent obtained from children and adolescents ≥11 years of age.
Participants
A total of 269 patients between 7 and 17 years of age with a primary Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) diagnosis of OCD were included in Step 1 (American Psychiatric Association 1994). Clinical response was defined as a CY-BOCS total score of ≤15 at posttreatment assessment by IEs. Based on this criterion, 27.4% (n=66) of the original 269 patients were determined to be nonresponders (Torp et al. 2015b). Twelve patients (18%) did not accept randomization (or dropped out before randomization). A total of 54 patients were available to be randomized to SRT or continued CBT. Because of treatment delay of >3 weeks, four patients assigned to SRT were reevaluated for eligibility. These four patients scored <16 on the CY-BOCS reassessment, and were, therefore, considered to be Step 1 treatment responders and not included in the Step 2 intent-to-treat sample. In total, 28 patients were allocated to continued CBT and 22 were allocated to SRT. Seven patients dropped out of each treatment arm, and the difference was not significant (χ2[1, 49]=0.046, p=0.830). All SRT participants who dropped out did so because they were opposed to medication use for pediatric OCD. Six participants randomized to continued CBT withdrew consent for continued CBT, whereas one participant terminated treatment because of a somatic disease. The attrition rate was also similar across tic disorders and treatment assignments. Among participants with tic disorder, two (28.6%) dropped out of CBT and one (20.0%) dropped out of SRT. Five (23.8%) participants without comorbid tic disorder dropped out of CBT and six (35.3%) dropped out of SRT.
Treatments
Step 2 SRT included six treatment visits over 16 weeks. SRT was chosen because it is the only SSRI approved for OCD treatment in children and adolescents in Denmark, Sweden, and Norway (Thomsen et al. 2013). The pharmacotherapy treatment manual was adapted from the manual used in the POTS study (2004). A starting dose of 25 mg/day was titrated up to 100 mg/day by week 4. Children <10 years of age with low body weight could be started on a lower dose as clinically indicated. If a participant's response was considered inadequate at a dose of 100 mg/day, the dose was increased gradually up to a maximum of 200 mg per day by week 8. Treatment response and adverse effects (AEs) were monitored at every visit, and the dose reduced if necessary. The manual also included guidelines for clinical support in which participants were encouraged to practice exposure tasks learned during Step 1 treatment. However, introducing new exposure tasks during Step 2 SRT treatment was not allowed. The overall rationale for this component was to reduce the variability of nonspecific treatment effects unrelated to SRT.
Participants randomized to continued CBT received 10 additional treatment sessions over 16 weeks. The same CBT principles used in Step 1 were used in Step 2. However, therapists were encouraged to identify and address barriers that may have interfered with treatment received in Step 1. Common factors identified were: 1) It took too long to engage the patient in exposure exercises because of high levels of anxiety or low motivation, 2) family factors, such as high initial accommodation, made exposure homework difficult, 3) in-office exposures did not generate high enough anxiety levels to be helpful, and 4) the participant's own expectation for improvement was low, and less effort was exerted during exposure exercises. Based on these and other factors, Step 2 CBT was tailored to address the specific problems unique to each individual case. For example, for Step 1 participants whose onset of exposure exercises was delayed, for whatever reason, an emphasis was placed on conducting more exposure exercises in Step 2 CBT. Or, for Step 1 participants whose OCD symptoms occurred primarily at home or at school, steps were taken to conduct in vivo exposures in the setting in which the symptoms most frequently occurred. Or, if family dynamics were seen as interfering with treatment progress, measures were taken to address these barriers, such as helping parents minimize accommodation to their child's OCD, decreasing OCD-related blame and guilt, and generating developmentally appropriate patterns of family interactions. The format of sessions in Step 2 CBT was the same as in Step 1 (Torp et al. 2015b). Sessions were scheduled for 90 minutes, in which the first hour centered on individual E/RP and the last half hour included one or both parents.
Measures
The Kiddie-Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime (K-SADS-PL) (Kaufman et al. 1997) is a semistructured diagnostic interview that assesses a variety of child psychopathology and demonstrates favorable psychometric properties. The K-SADS-PL has shown an excellent interrater reliability of 98% and a 1–5 week test–retest κ of 0.80 for any anxiety disorder diagnosis (Kaufman et al. 1997). Convergent and divergent validity of the K-SADS-PL have been documented in a Nordic sample of adolescents (Lauth et al. 2010). Moreover, the K-SADS-PL has been used in previous OCD treatment trials (Valderhaug et al. 2007; Freeman et al. 2008). Symptoms can be classified as “not present,” “possible,” “in remission,” or “certain.” In this study, OCD diagnoses and comorbidity were based on symptoms classified as “certain” only. The K-SADS-PL was used for diagnostic assessment at baseline of Step 1. All interviews were conducted by experienced clinicians, trained by the NordLOTS research group. Tic disorders included Tourette's disorder and chronic motor or vocal tic disorders as defined by the K-SADS-PL.
The CY-BOCS (Goodman et al. 1989) is a widely used clinician-rated, semistructured interview assessing OCD symptomatology. The CY-BOCS evaluates the severity of obsessions and compulsions, using 10 items across five dimensions (time occupied by symptoms, interference, distress, resistance, and degree of control over symptoms). The total severity score can range from 0 to 40. CY-BOCS total scores of 10–18 are considered mild, 19–28 are considered moderate, and ≥29 are considered severe (March et al. 1998). CY-BOCS shows reasonable reliability and validity (Scahill et al. 1997; Storch et al. 2004; Gallant et al. 2008). In particular, high internal consistency for the total score 0.87 (Scahill et al. 1997), and good to excellent interrater agreement have been reported (Scahill et al. 1997), (0.84, 0.91, and 0.68 for total score, obsessions, and compulsions, respectively). In the NordLOTS sample, the interrater agreement was 0.92 (95% CI 0.78–0.97), 0.94 (95% CI 0.85–0.97), and 0.87 (95% CI 0.67–0.93) for total score, obsessions, and compulsions, respectively. The CY-BOCS was used for assessment of treatment response at baseline, week 7 and week 13 in Step 1 and week 22 and week 30 in Step 2. The primary outcomes were CY-BOCS total score over 16 weeks, and binary clinical response defined as CY-BOCS≤15.
Statistical analysis
Data were analyzed using piecewise regression (Ryan and Porth 2007) or were sometimes referred to as a longitudinal discontinuity model (Singer and Willett 2003). This model, herein called the “longitudinal discontinuity model,” can be used to evaluate whether a shift in outcome trajectories occur following the occurrence of a known event. In this article, the known event is the onset of Step 2 treatment. That is, the longitudinal discontinuity model was used to evaluate whether the introduction of SRT in CBT nonresponders would impact the symptom trajectory more than continuing CBT in participants with and without comorbid tic disorder.
In order to evaluate a longitudinal discontinuity model, a point in time along the outcome trajectory of each participant was specified that marked the onset of when a given event occurred. A significant change in elevation and slope following the onset of Step 2 treatments suggests that the newer treatments were having an impact on the Step 2 outcomes above what would be expected if no Step 2 treatments had been provided.
The “baseline” longitudinal discontinuity model (against which the impact of Step 2 treatments was evaluated) included two random effects (intercept and days since baseline) and the following fixed effects: Binary indicators for site ([grand mean centered] Aarhus, Southern and Eastern Norway, Central Norway, Stockholm) with Gothenburg serving as the reference category. To this baseline model, a series of discontinuous multilevel models for change were fitted to the data using restricted maximum likelihood estimation. The outcome for each model was the CY-BOCS total score. Changes in elevation and slope following the introduction of Step 2 treatments were evaluated by introducing a level 1 individual growth parameter to the baseline model. The binary variable TREATMENT was allowed to vary across subjects and was used to mark the onset of Step 2 treatments. This variable can be thought of as a new intercept for the second level 1 individual growth trajectory, and if found to be statistically significant, suggests that the addition of continued CBT or SRT resulted in a shift (either up or down) in the average outcome trajectory. A second level 1 individual growth parameter POSTDAYS, which was also a time-varying predictor and allowed to vary across subjects, marked the passage of time following receipt of Step 2 treatments. This variable, if found to be statistically significant, suggests that the average slope (i.e., rate of change) following receipt of Step 2 treatments was different than the slope during the period preceding the onset of Step 2 treatments. In other words, it captures the additive effect of the new treatments on the outcome trajectory after receipt of a given treatment.
To evaluate whether the discontinuity varied by participants with or without comorbid tic disorder (TICS), models included an interaction term between discontinuity predictors (DAYS and POSTDAYS) and a level 2 predictor TICS. In addition, to evaluate whether the discontinuity varied significantly between participants (i.e., the magnitude of the discontinuity in slope was not the same for all participants), models in which DAYS and POSTDAYS were treated as random effects were also evaluated. To evaluate whether the addition of random effects for this parameter resulted in a better fitting model, the change in the deviance statistic relative to the model without additional random effect was used. This difference follows a χ2 distribution with degrees of freedom equal to the difference in the number of parameters between the two models. In all models, residual error terms were assumed to follow a mean-zero, normal distribution with an unstructured covariance structure used to capture the within person correlation over time. Tests were two tailed, and a p value of<0.05 was considered to indicate statistical significance. Models were fit using PROC MIXED in SAS Statistical Software, Version 9.4 (SAS Institute, Cary, NC).
Results
Twelve out of 50 patients (24.0%) were diagnosed with current Tourette's disorder or chronic vocal or motor tic disorder according to K-SADS-PL. Ten participants had Tourette's disorder (five in each treatment condition) and two had chronic tic disorder (both in continued CBT). The mean CY-BOCS total score at baseline in Step 1 (week 0) and Step 2 (week 13) was not significantly different between patients with a tic disorder (Step 1, mean=26.5, SD=5.7; Step 2, mean=20.6, SD=3.4) and without a tic disorder (Step 1, mean=26.2, SD=5.4; Step 2, 23.8, SD=4.2). The presence of a tic disorder was close to proportional between continued CBT (n=7, 25%) and SRT (n=5, 22.7%). We analyzed whether these groups had different OCD symptom profiles by looking at the CY-BOCS item lists of obsessions and compulsions. Repeated and ordering compulsions were more prevalent in the comorbid tic disorder group (χ2[49]=4.612, p=0.032; χ2[49]=3.944, p=0.047). We also saw a trend that hoarding compulsions were more prevalent in the tic disorder group (χ2[49]=3.791, p=0.052). All other comparisons were not significant. There were some indications of different comorbidity patterns, as 34.2% (n=13) of non-tic patients and only 8.3% (n=1) of tic patients were diagnosed with a comorbid internalizing disorders. However, this difference was however not statistically significant (χ2[49]=3.029, p=0.082). Externalizing disorders were overrepresented among tics patients (50.0%, n=6) compared with non-tic patients ([5.3%, n=2] χ2[49]=13.581, p<0.001).
Figure 1 displays the estimated population mean CY-BOCS growth curve trajectory over time for participants with and without tic disorder. The final model suggests that before onset of SRT, the average weekly rate of improvement on the CY-BOCS was −0.287 for participants without a comorbid tic disorder and −0.147 for participants with a comorbid tic disorder, which was not significantly different. This suggests that the rate of change during Step 1 treatments was the same for those subjects with and without a comorbid tic disorder. However, as is illustrated in Figure 1, at the onset of Step 2 treatments, those subjects who received SRT were 0.810 points higher on the CY-BOCS relative to those who received continued CBT. The average weekly rate of improvement increased to −0.293 for those without a comorbid tic disorder (2.2% faster than the corresponding rate during Step 1) and −0.573 for those with comorbid tic disorder (290% faster than the corresponding rate during Step 1) (Table 1).
FIG. 1.
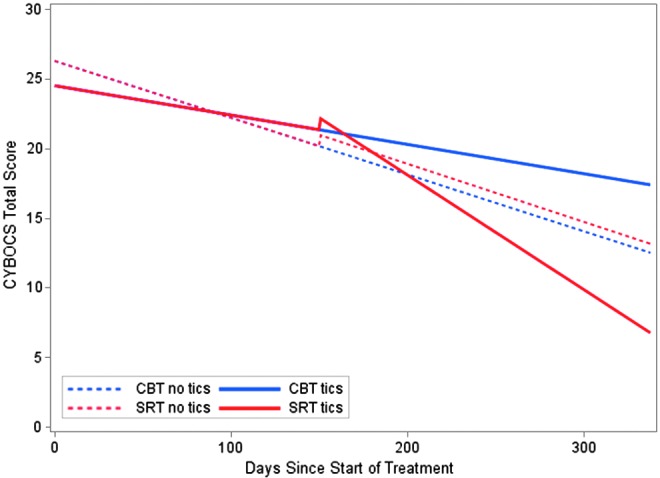
Adjusted intent-to-treat Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS) total score by days from baseline by treatment and presence or absence of tic disorder.
Table 1.
Parameter Estimates from Fitting Discontinuities in Elevation and Slope to the CY-BOCS
| Parameter | Baseline model | Final model | |
|---|---|---|---|
| Fixed effects | |||
| Composite model | Intercept | 21.581*** (0.574) | 26.284*** (0.920) |
| DAYS | −0.041*** (0.006) | ||
| TREATMENT (SRT) | 0.810 (0.970) | ||
| POSTDAYS | −0.0009 (0.011) | ||
| TICS | −1.773 (1.848) | ||
| TICS*DAYS | 0.020 (0.011) | ||
| TICS*POSTDAYS | −0.060* (0.023) | ||
| Aarhus vs. Gothenburg | 0.394 (0.968) | 0.409 (0.972) | |
| Eastern Norway vs. Gothenburg | 2.408 (1.241) | 2.095 (1.245) | |
| Central Norway vs. Gothenburg | 0.800 (0.832) | 0.995 (0.851) | |
| Stockholm vs. Gothenburg | −3.285* (1.373) | −3.385*** (1.389) | |
| Variance components | |||
| Level 1 | Within person | 11.230*** (1.422) | 10.848*** (1.380) |
| Level 2 | In initial status | 41.497*** (12.412) | 22.756*** (6.328) |
| In rate of change (days) | 0.002*** (0.001) | 0.0008*** (0.0002) | |
| Goodness-of-fit | |||
| Δ −2 Log likelihood | NA | 25.9 | |
| Δ df | NA | 6 | |
| p value | NA | <0.001 | |
p<0.05, ****p<0.001.
CY-BOCS, Children's Yale-Brown Obsessive Compulsive Scale; SRT, sertraline.
Planned pairwise comparisons of the average CY-BOCS score at posttreatment showed that continued CBT and SRT did not differ (t[118]=0.28, p=0.783) for patients without comorbid tic disorder. However, in patients with a comorbid tic disorder, the SRT group had significantly lower CY-BOCS total scores at posttreatment (t[118]=−2.68, p=0.009) than the continued CBT group (Table 2).
Table 2.
Group-Specific Predicted CY-BOCS Scores at Posttreatment
| End of Step 1 (95% CI) | End of Step 2 (95% CI) | |
|---|---|---|
| With tics | ||
| Continued CBT | 21.3 (18.9–23.7) | 17.40 (12.45–22.34) |
| Sertraline | 22.1 (19.4–24.9) | 6.78 (−0.49–14.05) |
| Without tics | ||
| Continued CBT | 20.1 (18.7–21.5) | 13.17 (8.95–17.38) |
| Sertraline | 20.9 (19.0–22.9) | 12.53 (9.61–15.44) |
CY-BOCS, Children's Yale-Brown Obsessive Compulsive Scale; CBT, cognitive-behavioral therapy.
Because internalizing disorders were overrepresented in patients without tics, and externalizing disorders were overrepresented in patients with tics, an unplanned sensitivity analysis was conducted by including comorbid internalizing and externalizing disorders from the K-SADS-PL. Two separate analyses were conducted by introducing either the presence or absence of internalizing or externalizing disorders, and the interaction between this predictor and DAYS and POSTDAYS. Tics as a moderator survived in both analyses, for both internalizing disorders (t[123]=−2.56, p=0.012) and externalizing disorders (t[123]=−4.29, p<0.001).
Discussion
Previous studies have consistently indicated that the presence of tics does not impact the effect of CBT (Piacentini et al. 2002; Himle et al. 2003; March et al. 2007; Storch et al. 2008; Torp et al., 2015a). However, the results for the SSRI studies are more inconsistent. Some indicate a negative impact of TD (McDougle et al. 1993; Geller et al. 2003; March et al. 2007), whereas others do not report any difference across groups with or without comorbid TD (March et al. 1998; Husted et al. 2007; Conelea et al. 2014). The results of our study are in contrast to these earlier results. Among CBT nonresponders, SRT was superior to continued CBT in patients with comorbid tics, whereas there was no significant difference between treatments among patients without tics.
The underlying mechanism for this effect is not clear. Consistent with earlier findings, our hypothesis was that patients with OCD and comorbid tic disorder will more often have an additional externalizing disorder, whereas patients with OCD without comorbid tic disorder will more often have an internalizing disorder (Lewin et al. 2010). Controlling for internalizing disorders did not change the moderating effect of tic disorder. However, when externalizing disorders were introduced into the model, tic disorder still moderated the results in the same direction, but more extremely.
One preliminary speculation is that compared with the other SSRIs, SRT is more effective as an inhibitor of dopamine reuptake (Bolden-Watson and Richelson 1993; Gordon et al. 1998; Kitaichi et al. 2010). Tic-related OCD is possibly associated with increased dopaminergic dysregulation in the ventral striatum (including the head of the caudate nucleus) relative to non-tic-related OCD (Shapiro et al. 1989; McDougle, et al. 1994; Eichstedt and Arnold 2001). As a potential consequence, adults with OCD and comorbid tic disorders benefit more by supplementing fluoxetine with neuroleptics such as haloperidol (that exhibits high affinity dopamine D2 receptor antagonism), whereas the addition of haloperidol does not seem to benefit OCD patients without a comorbid tic disorder (Bloch et al. 2006). Therefore, another preliminary speculation based on our results is that the dopaminergic activity of SRT may facilitate improvement in OCD patients with comorbid tic disorders that have already received CBT.
In addition to affecting the symptoms of tic-related OCD, the serotoninergic and dopamine reuptake of the SRT might affect other mechanisms as well, in favor of tic-related OCD; for example, moderating executive function such as response inhibition. One feature of OCD and tics is poor response inhibition and impulse control, which most probably stem from dysfunctional frontostriatal loops in OCD and tic disorders (Aouizerate et al. 2004). Dopaminergic and serotoninergic neurons play a role in these loops by affecting other brain areas associated with OCD and tic disorders by modulating basal ganglia efferents (Baxter et al. 1996; Rosenberg and Keshavan 1998; Marsh et al. 2009; Leckman et al. 2010). Tic-related OCD might have increased response inhibition/impulse control dysfunction (Muller et al. 2003). Consequently, SRT might lead to additional benefits for tic-related OCD compared with non-tic-related OCD, offering some relief from symptoms of both tics and OCD through the combined dopamine and serotonin reuptake blocking properties.
Moreover, OCD with and without comorbid tic disorders are different in terms of OCD symptom categories. Studies have shown a lower frequency of contamination obsessions and washing/cleaning compulsions in patients with OCD and tics but a higher frequency of checking, counting, touching, and blinking compulsions (de Groot et al. 1994; Holzer et al. 1994; Leckman et al. 1994). The dopaminergic reuptake effect of the SRT might have affected the symptom categories differently; for example, whether checking, counting, touching, and blinking compulsions in patients with tic-related OCD could be more improved than symptoms of patients without comorbid tic disorder. However, the current sample is too small to allow for that kind of analysis.
The findings suggest that children and adolescents with OCD and comorbid tic disorders who did not respond to an ordinary CBT trial could have an advantage by switching to an SSRI. Also, the combination of CBT and SSRIs could be a favorable option, as the POTS study showed the superiority of combined CBT and SRT in treatment-naïve patients regardless of the presence or absence of tics (March et al. 2007). However, to conclude on this particular question, one would need to randomize CBT nonresponders to either sertraline treatment or combined CBT and sertraline.
Strengths and limitations
A strength of our study was the deliberate decision to stratify the randomization procedure on present or absence of tic disorder. This helped to ensure a balanced number of patients with comorbid tics in both Step 2 treatment groups. Furthermore, instruments with well-reported psychometric properties were used for assessment. A limitation of this trial is that active treatments were not compared with a placebo control. Therefore, the absolute effect of SRT or continued CBT is not known. A future trial with a placebo control condition will be needed to rule out possible carry-over effects. Moreover, the study was based on a small sample size. However, it takes a great effort to study CBT nonresponders, and it is still not common to have such a large CBT nonresponder sample randomized to two different treatments.
The attrition rate was high: 28% across both treatment groups. All SRT participants who withdrew their consent did so because the parents did not want their children taking medication. Six patients withdrew consent because they did not want further CBT. High attrition rates between steps seem to be a common problem in stepped care studies (Gilliam et al. 2010; Tolin et al. 2011).
Conclusions
In conclusion, SRT was significantly superior to continued CBT in CBT nonresponders with a comorbid tic disorder, whereas patients without tic disorders showed no significant differences between treatments. These findings are in contrast to previous studies, and indicate that CBT nonresponders with OCD and comorbid tic disorders can benefit by switching to SSRI.
Clinical Significance
The data suggest that children and adolescents with OCD and comorbid tic disorders might benefit from SRT when they do not improve adequately with CBT.
Acknowledgments
Funding was applied to each national site as well as some central funding. We thank the following for their contributions: Trygfonden, The Danish Council for Strategic Research, Pulje til styrkelse af psykiatrisk forskning i Region Midtjylland, The Center for Child and Adolescent Mental Health, Eastern and Southern Norway (RBUP), Stiftelsen Clas Groschinskys Minnesfond, Norwegian Research Council, and Norwegian ExtraFoundation. We also thank all the clinics in our participating countries who donated time for assessments.
Disclosures
Dr. Compton has received grant support from the National Institutes of Health (NIH) and Shire. He is an associate editor at Journal of Consulting and Clinical Psychology, Journal of Child and Adolescent Psychopharmacology, and BMC Psychiatry. Dr. Ivarsson is a consultant and on a speakers' bureau for Shire, Sweden. The other authors have nothing to disclose.
References
- Abramowitz J, Whiteside S, Deacon B: The effectiveness of treatment for pediatric obsessive-compulsive disorder: A meta-analysis. Behav Ther 36:55–63, 2005 [Google Scholar]
- American Academy of Child and Adolescent Psychiatry: Practice parameter for the assessment and treatment of children and adolescents with obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry. 51:98–113, 2012 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association.:Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013 [Google Scholar]
- Aouizerate B, Guehl D, Cuny E, Rougier A, Bioulac B, Tignol J, Burbaud P: Pathophysiology of obsessive-compulsive disorder: A necessary link between phenomenology, neuropsychology, imagery and physiology. Prog Neurobiol 72:195–221, 2004 [DOI] [PubMed] [Google Scholar]
- Baxter LR, Jr., Saxena S, Brody AL, Ackermann RF, Colgan M, Schwartz JM, Allen–Martinez Z, Fuster JM, Phelps ME: Brain mediation of obsessive-compulsive disorder symptoms: Evidence from functional brain imaging studies in the human and nonhuman primate. Semin Clin Neuropsychiatry 1:32–47, 1996 [DOI] [PubMed] [Google Scholar]
- Bloch MH, Landeros–Weisenberger A, Kelmendi B, Coric V, Bracken MB, Leckman JF: A systematic review: Antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 11:622–632, 2006 [DOI] [PubMed] [Google Scholar]
- Bolden–Watson C, Richelson E: Blockade by newly-developed antidepressants of biogenic amine uptake into rat brain synaptosomes. Life Sci 52:1023–1029, 1993 [DOI] [PubMed] [Google Scholar]
- Cath DC, Spinhoven P, van Woerkom TC, van de Wetering BJ, Hoogduin CA, Landman AD, Roos RA, Rooijmans HG: Gilles de la Tourette's syndrome with and without obsessive-compulsive disorder compared with obsessive-compulsive disorder without tics: which symptoms discriminate? J Nerv Ment Dis 189:219–228, 2001 [DOI] [PubMed] [Google Scholar]
- Coffey BJ, Miguel EC, Biederman J, Baer L, Rauch SL, O'Sullivan RL, Savage CR, Phillips K, Borgman A, Green–Leibovitz MI, Moore E, Park KS, Jenike MA: Tourette's disorder with and without obsessive-compulsive disorder in adults: are they different? J Nerv Ment Dis 186:201–206, 1998 [DOI] [PubMed] [Google Scholar]
- Conelea CA, Walther MR, Freeman JB, Garcia AM, Sapyta J, Khanna M, Franklin M: Tic-related obsessive-compulsive disorder (OCD): Phenomenology and treatment outcome in the pediatric OCD treatment study II. J Am Acad Child Adolesc Psychiatry 53:1308–1316, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot CM, Bornstein RA, Janus MD, Mavissakalian MR: Patterns of obsessive compulsive symptoms in Tourette subjects are independent of severity. Anxiety 1:268–274, 1994 [DOI] [PubMed] [Google Scholar]
- Eichstedt JA, Arnold SL: Childhood-onset obsessive-compulsive disorder: A tic-related subtype of OCD? Clin Psychol Rev 21:137–157, 2001 [DOI] [PubMed] [Google Scholar]
- Freeman JB, Garcia AM, Coyne L, Ale C, Przeworski A, Himle M, Compton S, Leonard HL: Early childhood OCD: Preliminary findings from a family-based cognitive-behavioral approach. J Am Acad Child Adolesc Psychiatry 47:593–602, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant J, Storch EA, Merlo LJ, Ricketts ED, Geffken GR, Goodman WK, Murphy TK: Convergent and discriminant validity of the Children's Yale-Brown Obsessive Compulsive Scale–Symptom Checklist. J Anxiety Disord 22:1369–1376, 2008 [DOI] [PubMed] [Google Scholar]
- Garcia AM, Sapyta JJ, Moore PS, Freeman JB, Franklin ME, March JS, Foa EB: Predictors and moderators of treatment outcome in the Pediatric Obsessive Compulsive Treatment Study (POTS I). J Am Acad Child Adolesc Psychiatry 49:1024–1033; quiz 1086, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller DA, Biederman J, Stewart SE, Mullin B, Farrell C, Wagner KD, Emslie G, Carpenter D: Impact of comorbidity on treatment response to paroxetine in pediatric obsessive-compulsive disorder: Is the use of exclusion criteria empirically supported in randomized clinical trials? J Child Adolesc Psychopharmacol 13 Suppl 1:S19–29, 2003 [DOI] [PubMed] [Google Scholar]
- Gilliam CM, Diefenbach GJ, Whiting SE, Tolin DF: Stepped care for obsessive-compulsive disorder: An open trial. Behav Res Ther 48:1144–1149, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg GS, Kingery JN, Drake KL, Grados MA: Predictors of treatment response in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 47:868–878, 2008 [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS: The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46:1006–1011, 1989 [DOI] [PubMed] [Google Scholar]
- Gordon C, Whale R, Cowen PJ: Sertraline treatment does not increase plasma prolactin levels in healthy subjects. Psychopharmacology (Berl) 137:201–202, 1998 [DOI] [PubMed] [Google Scholar]
- Hanna GL: Demographic and clinical features of obsessive-compulsive disorder in children and adolescents. J Am Acad Child Adolesc Psychiatry 34:19–27, 1995 [DOI] [PubMed] [Google Scholar]
- Himle JA, Fischer DJ, Van Etten ML, Janeck AS, Hanna GL: Group behavioral therapy for adolescents with tic-related and non-tic-related obsessive-compulsive disorder. Depress Anxiety 17:73–77, 2003 [DOI] [PubMed] [Google Scholar]
- Holzer JC, Goodman WK, McDougle CJ, Baer L, Boyarsky BK, Leckman JF, Price LH: Obsessive-compulsive disorder with and without a chronic tic disorder. A comparison of symptoms in 70 patients. Br J Psychiatry 164:469–473, 1994 [DOI] [PubMed] [Google Scholar]
- Husted DS, Shapira NA, Murphy TK, Mann GD, Ward HE, Goodman WK: Effect of comorbid tics on a clinically meaningful response to 8-week open-label trial of fluoxetine in obsessive compulsive disorder. J Psychiatr Res 41:332–337, 2007 [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Melin K, Wallin L: Categorical and dimensional aspects of co-morbidity in obsessive-compulsive disorder (OCD). Eur Child Adolesc Psychiatry 17:20–31, 2008 [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Skarphedinsson G, Kornør H, Axelsdottir B, Biedilæ S, Heyman I, Asbahr FR, Thomsen P, Fineberg NA, March J: Selective reuptake inhibitors (SRIs) for obsessive compulsive disorder (OCD) in children and adolescents: A systematic review and meta-analysis. Psychiatr Res In press [DOI] [PubMed] [Google Scholar]
- Ivarsson T, Thomsen PH, Dahl K, Valderhaug R, Weidle B, Nissen JB, Englyst I, Christensen K, Torp NC, Melin K: The rationale and some features of the Nordic Long-Term OCD Treatment Study (NordLOTS) in childhood and adolescence. Child Youth Care Forum 39:91–99, 2010 [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T, Koyama T: Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. Eur J Pharmacol 647:90–96, 2010 [DOI] [PubMed] [Google Scholar]
- Lauth B, Arnkelsson GB, Magnusson P, Skarphedinsson G, Ferrari P, Petursson H: Validity of K-SADS-PL (Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version) depression diagnoses in an adolescent clinical population. Nord J Psychiatry 64:409–420, 2010 [DOI] [PubMed] [Google Scholar]
- Leckman JF, Bloch MH, Smith ME, Larabi D, Hampson M: Neurobiological substrates of Tourette's disorder. J Child Adolesc Psychopharmacol 20:237–247, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Grice DE, Barr LC, de Vries AL, Martin C, Cohen DJ, McDougle CJ, Goodman WK, Rasmussen SA: Tic-related vs. non-tic-related obsessive compulsive disorder. Anxiety 1:208–215, 1994 [PubMed] [Google Scholar]
- Leonard HL, Lenane MC, Swedo SE, Rettew DC, Gershon ES, Rapoport JL: Tics and Tourette's disorder: A 2- to 7-year follow-up of 54 obsessive-compulsive children. Am J Psychiatry 149:1244–1251, 1992 [DOI] [PubMed] [Google Scholar]
- Lewin AB, Chang S, McCracken J, McQueen M, Piacentini J: Comparison of clinical features among youth with tic disorders, obsessive-compulsive disorder (OCD), and both conditions. Psychiatry Res 178:317–322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS, Biederman J, Wolkow R, Safferman A, Mardekian J, Cook EH, Cutler NR, Dominguez R, Ferguson J, Muller B, Riesenberg R, Rosenthal M, Sallee FR, Wagner KD, Steiner H: Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 280:1752–1756, 1998 [DOI] [PubMed] [Google Scholar]
- March JS, Franklin ME, Leonard H, Garcia A, Moore P, Freeman J, Foa E: Tics moderate treatment outcome with sertraline but not cognitive-behavior therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry 61:344–347, 2007 [DOI] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. Am J Psychiatry 166:664–674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougle CJ, Goodman WK, Leckman JF, Barr LC, Heninger GR, Price LH: The efficacy of fluvoxamine in obsessive-compulsive disorder: Effects of comorbid chronic tic disorder. J Clin Psychopharmacol 13:354–358, 1993 [PubMed] [Google Scholar]
- McDougle CJ, Goodman WK, Leckman JF, Lee NC, Heninger GR, Price LH: Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder. A double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 51:302–308, 1994 [DOI] [PubMed] [Google Scholar]
- Muller SV, Johannes S, Wieringa B, Weber A, Muller–Vahl K, Matzke M, Kolbe H, Dengler R, Munte TF: Disturbed monitoring and response inhibition in patients with Gilles de la Tourette syndrome and co-morbid obsessive compulsive disorder. Behav Neurol 14:29–37, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, 2nd, Goodman W, Rasmussen S, Leckman JF: A family study of obsessive-compulsive disorder. Am J Psychiatry 152:76–84, 1995 [DOI] [PubMed] [Google Scholar]
- Pediatric OCD Treatment Study Team: Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: The Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA 292:1969–1976, 2004 [DOI] [PubMed] [Google Scholar]
- Peterson BS, Pine DS, Cohen P, Brook JS: Prospective, longitudinal study of tic, obsessive-compulsive, and attention-deficit/hyperactivity disorders in an epidemiological sample. J Am Acad Child Adolesc Psychiatry 40:685–695, 2001 [DOI] [PubMed] [Google Scholar]
- Piacentini J, Bergman RL, Jacobs C, McCracken JT, Kretchman J: Open trial of cognitive behavior therapy for childhood obsessive-compulsive disorder. J Anxiety Disord 16:207–219, 2002 [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Inoff–Germain G, Weissman MM, Greenwald S, Narrow WE, Jensen PS, Lahey BB, Canino G: Childhood obsessive-compulsive disorder in the NIMH MECA study: Parent versus child identification of cases. Methods for the epidemiology of child and adolescent mental disorders. J Anxiety Disord 14:535–548, 2000 [DOI] [PubMed] [Google Scholar]
- Riddle MA, Scahill L, King R, Hardin MT, Towbin KE, Ort SI, Leckman JF, Cohen DJ: Obsessive compulsive disorder in children and adolescents: Phenomenology and family history. J Am Acad Child Adolesc Psychiatry 29:766–772, 1990 [DOI] [PubMed] [Google Scholar]
- Rosenberg DR, Keshavan MS: A.E. Bennett Research Award. Toward a neurodevelopmental model of of obsessive–compulsive disorder. Biol Psychiatry 43:623–640, 1998 [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Stein DJ, Chiu WT, Kessler RC. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15:53–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SE, Porth LS: A tutorial on the piecewise regression approach applied to bedload transport data. Gen. Tech. Rep. RMRS-GTR-189. Fort Collins, CO: Department of Agriculture FS, Rocky Mountain Research Station; 2007 [Google Scholar]
- Scahill L, Riddle MA, McSwiggin–Hardin M, Ort SI: Children's Yale-Brown Obsessive Compulsive Scale: Reliability and validity. J Am Acad Child Adolesc Psychiatry 36:844–852, 1997 [DOI] [PubMed] [Google Scholar]
- Shapiro E, Shapiro AK, Fulop G, Hubbard M, Mandeli J, Nordlie J, Phillips RA: Controlled study of haloperidol, pimozide and placebo for the treatment of Gilles de la Tourette's syndrome. Arch Gen Psychiatry 46:722–730, 1989 [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB: Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford: Oxford University Press; 2003 [Google Scholar]
- Skarphedinsson G, Hanssen–Bauer K, Kornor H, Heiervang ER, Landro NI, Axelsdottir B, Biedilae S, Ivarsson T: Standard individual cognitive behaviour therapy for paediatric obsessive-compulsive disorder: A systematic review of effect estimates across comparisons. Nord J Psychiatry 69:81–92, 2015 [DOI] [PubMed] [Google Scholar]
- Skarphedinsson G, Weidle B, Thomsen P, Dahl K, Torp NC, Nissen JB, Melin K, Hybel K, Valderhaug R, Wentzel–Larsen T, Ivarsson T: Continued cognitive behavior therapy versus sertraline for non- or partial responders to cognitive behavior therapy: A randomized controlled trial. Presented at the 60th Annual Meeting of the American Academy of Child and Adolescent Psychiatry, Orlando, FL, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Larson MJ, Geffken GR, Lehmkuhl HD, Jacob ML, Murphy TK, Goodman WK: Impact of comorbidity on cognitive-behavioral therapy response in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 47:583–592, 2008 [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Geffken GR, Soto O, Sajid M, Allen P, Roberti JW, Killiany EM, Goodman WK: Psychometric evaluation of the Children's Yale-Brown Obsessive-Compulsive Scale. Psychiatry Res 129:91–98, 2004 [DOI] [PubMed] [Google Scholar]
- Thomsen PH, Mikkelsen HU: Course of obsessive-compulsive disorder in children and adolescents: a prospective follow-up study of 23 Danish cases. J Am Acad Child Adolesc Psychiatry 34:1432–1440, 1995 [DOI] [PubMed] [Google Scholar]
- Thomsen PH, Torp NC, Dahl K, Christensen K, Englyst I, Melin KH, Nissen JB, Hybel KA, Valderhaug R, Weidle B, Skarphedinsson G, von Bahr PL, Ivarsson T: The Nordic Long-Term OCD Treatment Study (NordLOTS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health 7:41, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin DF, Diefenbach GJ, Gilliam CM: Stepped care versus standard cognitive-behavioral therapy for obsessive-compulsive disorder: A preliminary study of efficacy and costs. Depress Anxiety 28:314–323, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro J, Cervera M, Osejo E, Salamero M: Obsessive-compulsive disorder in childhood and adolescence: a clinical study. J Child Psychol Psychiatry 33:1025–1037, 1992 [DOI] [PubMed] [Google Scholar]
- Torp NC, Dahl K, Skarphedinsson G, Thomsen PH, Valderhaug R, Weidle B, Hybel K, Compton S, Ivarsson T: Predictors associated with improved cognitive-behavioral therapy outcome in pediatric obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 54:200–207, 2015a [DOI] [PubMed] [Google Scholar]
- Torp NC, Dahl K, Skarphedinsson G, Thomsen P, Valderhaug R, Weidle B, Melin K, Hybel K, Nissen JB, Lenhard F, Wentzel–Larsen T, Franklin M, Ivarsson T: Effectiveness of cognitive behavior treatment for pediatric obsessive-compulsive disorder: Acute outcomes from the Nordic Long-term OCD Treatment Study (NordLOTS). Behav Res Ther 64:15–23, 2015 [DOI] [PubMed] [Google Scholar]
- Valderhaug R, Larsson B, Gotestam KG, Piacentini J: An open clinical trial of cognitive-behaviour therapy in children and adolescents with obsessive-compulsive disorder administered in regular outpatient clinics. Behav Res Ther 45:577–589, 2007 [DOI] [PubMed] [Google Scholar]
- Watson HJ, Rees CS: Meta-analysis of randomized, controlled treatment trials for pediatric obsessive-compulsive disorder. J Child Psychol Psychiatry 49:489–498, 2008 [DOI] [PubMed] [Google Scholar]
- Zohar AH, Ratzoni G, Pauls DL, Apter A, Bleich A, Kron S, Rappaport M, Weizman A, Cohen DJ: An epidemiological study of obsessive-compulsive disorder and related disorders in Israeli adolescents. J Am Acad Child Adolesc Psychiatry 31:1057–1061, 1992 [DOI] [PubMed] [Google Scholar]


