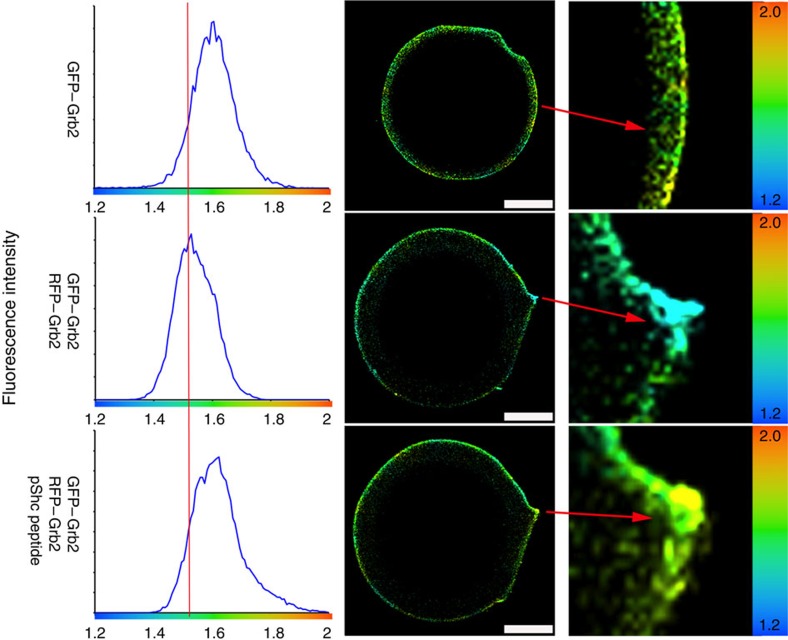
Figure 3. Binding of phosphotyrosine by Grb2-SH2 domain leads to dissociation.
smFLIM shows Grb2 dimerization is disrupted upon phospho-Shc peptide binding by its SH2 domain. GFP-tagged Grb2 immobilized on GFP-trap beads were imaged in 20 mM Tris-HCl, 50 mM NaCl at pH 8.0. The reference average lifetime in the absence of acceptor is 1.6 ns. RFP–Grb2 was added and allowed to form complex for 1 h and the lifetime of GFP–Grb2 shortens as indicated by left-shifted peak with average lifetime centred on 1.5 ns clearly indicating that FRET has occurred. Phospho-Shc peptide (10 μM) containing the pYxN motif was then added, allowed to equilibrate for an hour and the fluorescence lifetime was measured. Addition of the phospho-Shc peptide restores the lifetime to the control values obtained in the absence of acceptor. This clearly shows that the phospho-Shc peptide disrupts dimerization of Grb2. The zoomed image of the bead (right hand column) shows lifetime values mapped to a false colour image. The change in fluorescence lifetime as a function of colour is highly noticeable. The data presented here was consistently reproduced in three independent experiments. Scale bars, 10 μm.