Abstract
Background
Vitamin D is associated with lung function in cross-sectional studies, and vitamin D inadequacy is hypothesized to play a role in the pathogenesis of chronic obstructive pulmonary disease. Further data are needed to clarify the relation between vitamin D status, genetic variation in vitamin D metabolic genes, and cross-sectional and longitudinal changes in lung function in healthy adults.
Methods
We estimated the association between serum 25-hydroxyvitamin D [25(OH)D] and cross-sectional forced expiratory volume in the first second (FEV1) in Framingham Heart Study (FHS) Offspring and Third Generation participants and the association between serum 25(OH)D and longitudinal change in FEV1 in Third Generation participants using linear mixed-effects models. Using a gene-based approach, we investigated the association between 241 SNPs in 6 select vitamin D metabolic genes in relation to longitudinal change in FEV1 in Offspring participants and pursued replication of these findings in a meta-analyzed set of 4 independent cohorts.
Results
We found a positive cross-sectional association between 25(OH)D and FEV1 in FHS Offspring and Third Generation participants (P = 0.004). There was little or no association between 25(OH)D and longitudinal change in FEV1 in Third Generation participants (P = 0.97). In Offspring participants, the CYP2R1 gene, hypothesized to influence usual serum 25(OH)D status, was associated with longitudinal change in FEV1 (gene-based P < 0.05). The most significantly associated SNP from CYP2R1 had a consistent direction of association with FEV1 in the meta-analyzed set of replication cohorts, but the association did not reach statistical significance thresholds (P = 0.09).
Conclusions
Serum 25(OH)D status was associated with cross-sectional FEV1, but not longitudinal change in FEV1. The inconsistent associations may be driven by differences in the groups studied. CYP2R1 demonstrated a gene-based association with longitudinal change in FEV1 and is a promising candidate gene for further studies.
Electronic supplementary material
The online version of this article (doi:10.1186/s12931-015-0238-y) contains supplementary material, which is available to authorized users.
Keywords: Vitamin D, 25-hydroxyvitamin D, FEV1, CYP2R1, Lung function, Framingham Heart Study
Introduction
Decreased lung function due to airflow obstruction is the primary characteristic of chronic obstructive pulmonary disease (COPD), the 3rd leading cause of mortality in the United States [1]. Vitamin D status, assessed by the circulating serum biomarker 25-hydroxyvitamin D [25(OH)D], plays a well-known role in bone health, and is also associated with non-skeletal outcomes including lung function [2, 3]. National surveys estimate that over 30 % of Americans are at risk for vitamin D insufficiency (defined as serum 25(OH)D <20 ng/mL) [3, 4]. Vitamin D is obtained through sun exposure and diet [2], and genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) in vitamin D metabolic genes that are significantly associated with serum 25(OH)D concentrations [5, 6].
The active vitamin D metabolite, 1,25(OH)2D, is constitutively synthesized from 25(OH)D in vitro in renal and extra-renal tissues including lung tissue [7] and is involved in biological processes critical to lung function including inflammation and airway remodeling [8–10]. Several cross-sectional, population-based observational studies have demonstrated strong, positive associations between vitamin D levels and lung function [11–13], although one study in the Hertfordshire cohort did not replicate cross-sectional associations [14]. Additionally, vitamin D deficiency is common in COPD patients [15], and high-dose vitamin D supplementation reduced COPD exacerbations in patients with severe vitamin D deficiency [16]. High vitamin D levels have also been associated with reduced risk of respiratory infections [13, 17], although randomized controlled trials of vitamin D supplementation and respiratory infections have been inconclusive [18, 19]. A recent cohort study demonstrated that low serum vitamin D was associated both with steeper lung function decline and a higher risk of developing COPD [20]. Additionally, an observational study in COPD patients reported no association between serum 25(OH)D and longitudinal lung outcomes [21], but a recent population-based study in an elderly male cohort reported steeper lung function decline in current smokers with serum 25(OH)D ≤20 ng/mL versus smokers with higher 25(OH)D [22]. Genetic variants in the vitamin D binding protein, encoded by the GC gene, are associated with COPD risk [15, 23–26], and GC may be an important mediator of hypothesized vitamin D effects on lung function [27].
Our study investigated the association of serum 25(OH)D with lung function in two generational cohorts of the Framingham Heart Study (FHS). Both serum measurements of vitamin D and genetic variants associated with serum vitamin D levels were investigated in association with cross-sectional FEV1 and rate of change in FEV1. Overall, this study provides a comprehensive exploration of serum 25(OH)D associations with FEV1 in a healthy, adult population-based sample including both men and women.
Methods
Study design
We investigated the association of serum 25(OH)D with lung function in two Framingham Heart Study (FHS) generational cohorts, the Offspring cohort and the Third Generation cohort. The availability of the specific data needed for the stages of the research was the main driver of which cohort(s) contributed to each analysis. We pursued replication of the SNP findings in four independent epidemiologic cohort studies, namely the Health, Aging and Body Composition Study (Health ABC), the Coronary Artery Risk Development in Young Adults Study (CARDIA), the Busselton Health Study (BHS), and the Cardiovascular Health Study (CHS), and we investigated the SNPs in relation to serum 25(OH)D status in the SUNLIGHT consortium [6].
Study population and ethics
Study participants were from the Offspring and Third Generation cohorts of the FHS, a longitudinal family-based study established in 1948 in Framingham, MA. The Offspring cohort, consisting of original cohort offspring and their spouses, began in 1971 [28]. The Third Generation cohort was initiated in 2002, enrolling children of the Offspring cohort [29]. Self-reported ethnicity across all FHS cohorts was >99 % Caucasian [29].
Third Generation participants with serum 25(OH)D measurements from Exam 1 (2002–2005) and spirometry measurements from Exams 1 and 2 (2008–2010) were included in all serum 25(OH)D—FEV1 analyses (N = 3,599; 88 % of full cohort). 1,435 Offspring participants (28 % of full cohort) had serum 25(OH)D and spirometry measurements at either Exam 6 (1995–1998) or 7 (1998–2001), and were included in the cross-sectional serum 25(OH)D—FEV1 analyses. The Offspring participants were not included in the serum 25(OH)D—rate of change in FEV1 analysis because only 24 % of the full Offspring cohort had repeated lung function measured after serum vitamin D, and we expected survivor bias to affect the longitudinal associations (see Results section). Serum vitamin D and pulmonary function were measured concurrently in Offspring participants, and thus the Offspring cohort data contributed to estimation of the cross-sectional association. If any bias exists, it is expected to be conservative (estimate closer to the null value) because the range in vitamin D may be restricted in the subsample with data. The SNP—rate of change in FEV1 associations were studied in 3,230 Offspring participants (63 % of full cohort) with GWAS genotype data and spirometry measurements spanning over Exams 5–8, which provided optimal information on longitudinal change in lung function given the number of repeated measurements and the length of time between measurements.
All study participants provided written informed consent for this study, and local institutional review boards approved the study protocols.
Measures
Genotyping was performed using the Affymetrix 500 K SNP array with a supplemental Affymetrix 50 K gene-focused array. Genotyping and imputation methods are described in detail elsewhere [6, 30]. 241 imputed SNPs in six candidate genes with well-established roles in vitamin D metabolism and transport [CYP24A1, CYP27A1, CYP27B1, CYP2R1, DHCR7/NADSYN1 (these two genes considered jointly as a candidate genomic locus due to prior GWAS associations [5, 6]), and GC] were analyzed (+/− 5 KB region on either end of genes included; Additional file 1: Table S1 for details).
Pulmonary function test measurements meeting American Thoracic Society/European Respiratory Society criteria for acceptability and reproducibility were used [31] (see Additional file 1 for further details).
Serum 25(OH)D was measured using radioimmunoassay (DiaSorin Inc, Stillwater, MN, USA) [6, 32, 33], and log-transformed values were used in all analyses (see Additional file 1 for further details). Offspring serum samples for 25(OH)D were collected between 1997–2001 [32] and Third Generation serum samples between 2001–2005 [6, 33]; assays were completed separately in different laboratories. However, all FHS samples were analyzed after 1998, so assay drifts due to the reformulated RIA assay, described for the NHANES data [34], are not expected to affect 25(OH)D measurements in Framingham (Additional file 1 for further quality control information on assays).
Smoking pattern was defined as: persistent smoker (current smoker, all time points during follow-up), intermittent smoker (current smoker at ≥ 1 time point), former smoker (former smoker, all time points), and never smoker (never smoker, all time points).
Statistical analysis
All analyses used linear mixed effect models with a random effect to account for familial correlation. The independent variable was FEV1; in serum 25(OH)D—rate of change in FEV1 analyses, the coefficient of interest was the interaction of 25(OH)D x time (time = time elapsed between the initial FEV1 measurement and each subsequent measurement), which estimated the effect of serum 25(OH)D on rate of change in FEV1. The cross-sectional association of serum 25(OH)D with FEV1 was estimated by the coefficient for serum 25(OH)D from the mixed effect models; all participants with serum 25(OH)D had a concurrent measurement of pulmonary function. In SNP—rate of change in FEV1 analyses, the coefficient for the interaction of SNP x time estimated the additive effect of the coded allele on rate of change in FEV1. All models were adjusted for baseline age, sex, height, current smoking status, smoking pattern during follow-up (defined in previous paragraph), the interaction of smoking pattern x time, and baseline pack-years. Serum 25(OH)D—FEV1 models were further adjusted for month of 25(OH)D measurement, body mass index (BMI), and FHS cohort (for models including both Offspring and Third Generation participants). Genetic models were further adjusted for the first two ancestry principal components to account for population substructure [35]. Analyses were completed using R (version 2.15.3).
A staged approach was used for SNP—FEV1 analyses. First, a gene-based P-value was calculated for the association of each candidate gene with rate of change in FEV1 using the VEGAS program [36, 37]. VEGAS calculates a single, gene-based test statistic based on P-values from the SNP—rate of change in FEV1 regression analysis across a given gene region, taking the linkage disequilibrium (LD) structure of the gene into account [36]. A gene-based P-value < 0.05 was chosen to select genes to follow-up in replication. Second, the most significant SNP from candidate genes with a gene-based P < 0.05 was assessed for replication in a meta-analysis of the four replication cohorts. The most significant SNP from each gene with a gene-based P < 0.05 was further evaluated for association with serum 25(OH)D using data from the SUNLIGHT consortium, which comprised 33,996 individuals of European ancestry, including FHS participants, with GWAS data and the serum 25(OH)D phenotype [6].
Results
Average serum 25(OH)D in the 28 % of the Offspring participants with data was 18.0 ng/mL, compared with 34.5 ng/mL in Third Generation participants; similarly, the proportion of participants at risk of vitamin D deficiency (defined as serum 25(OH)D <12 ng/mL) was 14.4 % and 1.2 % in the Offspring and Third Generation cohorts, respectively (Table 1). As expected, Offspring participants were older, had lower baseline FEV1, and were more likely to be former smokers than the Third Generation participants (Tables 1 and 2).
Table 1.
Baseline1 population characteristics of Framingham Heart Study participants
| 25(OH)D—FEV1 Analyses | Offspring Cohort | Third Generation Cohort | P-value2 |
|---|---|---|---|
| N in analysis | N = 1,435 | N = 3,599 | |
| FEV1, L | 2.7 (0.8) | 3.6 (0.8) | <0.0001 |
| Follow-up duration, yr3 | 7.2 (1.9) | 6.1 (0.6) | <0.0001 |
| Baseline age, yr | 59.9 (9.2) | 40.2 (8.7) | <0.0001 |
| Male, % | 48 | 47 | 0.84 |
| Height, cm | 168.0 (9.1) | 170.6 (9.3) | <0.0001 |
| Baseline pack-years | 26.0 (22.7) | 13.7 (14.2) | <0.0001 |
| Current smokers4, % | 12.8 | 15.2 | 0.02 |
| Former smokers4, % | 50.8 | 27.0 | <0.0001 |
| BMI | 28.0 (5.1) | 26.9 (5.4) | <0.0001 |
| 25(OH)D, ng/ml5 | 18.0 (1.5) | 34.5 (1.5) | <0.0001 |
| N at risk of 25(OH)D deficiency (<12 ng/mL), % | 207 (14.4 %) | 44 (1.2 %) | <0.0001 |
| N at risk of 25(OH)D inadequacy (<20 ng/mL), % | 801 (55.8 %) | 311 (8.6 %) | <0.0001 |
1Baseline measurements are from the exam at time of vitamin D measurement (either Exam 6 or 7 for Offspring, and Exam 1 for Third Generation); Participant characteristics listed as mean (SD) for continuous variables and number (%) for categorical variables
2 P-values for continuous variables calculated from unpaired t-test; P-values for categorical variables calculated from chi-square test
3Follow-up duration of spirometry measurements subsequent to serum 25(OH)D measurement; average duration calculated for N = 1,223 Offspring cohort participants with longitudinal spirometry and N = 2,894 Third Generation cohort participants
4Current smokers at baseline; former smokers at all time points
5Geometric mean of 25(OH)D
Table 2.
Baseline1 population characteristics of Framingham Heart Study participants contributing to SNP analysis
| SNP—FEV1 Analysis | Offspring Cohort |
|---|---|
| N in analysis | N = 3,230 |
| FEV1, L | 3.0 (0.8) |
| Follow-up duration, yr | 14.7 |
| Baseline age, yr | 50.9 (10.3) |
| Male, % | 47 |
| Height, cm | 165.5 (9.5) |
| Baseline pack-years | 25.4 (21.3) |
| Current smokers2, % | 24.6 |
| Former smokers2, % | 39.8 |
1 Baseline measurements refer to Exam 5; Participant characteristics listed as mean (SD) for continuous variables and number (%) for categorical variables
2 Current smokers at baseline; former smokers at all time points
25(OH)D associations with cross-sectional FEV1 and rate of change in FEV1
25(OH)D was positively associated with FEV1 in the cross-sectional analysis of the combined sample of Offspring and Third Generation participants, such that a 1-unit increase in log-transformed 25(OH)D was associated with a 45 mL increase in FEV1 (P = 0.004) (Table 3). A consistent direction of association was observed for the dichotomous vitamin D variable at thresholds of <12 ng/mL or <20 ng/mL, using the Institute of Medicine thresholds for risk of vitamin D deficiency and inadequacy, respectively [3], but coefficients for the dichotomous serum vitamin D variables did not reach the significance threshold of P < 0.05. The spline analysis estimating the cross-sectional serum 25(OH)D—FEV1 association showed an approximately linear, positive association for serum 25(OH)D < 12 ng/mL. In the 12 to 40 ng/mL range, the serum 25(OH)D—FEV1 association attenuated, and a plateau was reached above a threshold of about 40 ng/mL (Fig. 1). The pattern of association did not differ between Offspring and Third Generation participants (sub panels of Fig. 1), and the estimates of the linear serum 25(OH)D—FEV1 association were about the same in both cohorts (beta coefficient for serum 25(OH)D—FEV1 association was 37 (SE: 29, P = 0.20) and 34 (SE: 19, P = 0.08) mL in Offspring and Third Generation, respectively).
Table 3.
Cross-sectional multivariable association of 25(OH)D with baseline FEV1 (mL) in the Offspring and Third Generation cohorts, combined (N = 5,034)
| Model categorization of vitamin D: | β | SE | P |
|---|---|---|---|
| Continuous : Log-transformed 25(OH)D | 45.2 | 15.5 | 0.004 |
| Dichotomy 1: At risk of vitamin D deficiency (<12 ng/mL) vs. not | −46.7 | 26.7 | 0.08 |
| Dichotomy 2: At risk of vitamin D inadequacy (<20 ng/mL) vs. not | −30.6 | 16.6 | 0.07 |
Adjusted for: baseline age, sex, height, smoking pattern, current smoking status, baseline pack-years, FHS cohort, baseline BMI, and month of blood draw; all coefficients show expected direction of effect
β beta coefficient, SE standard error, P P-value
Fig. 1.
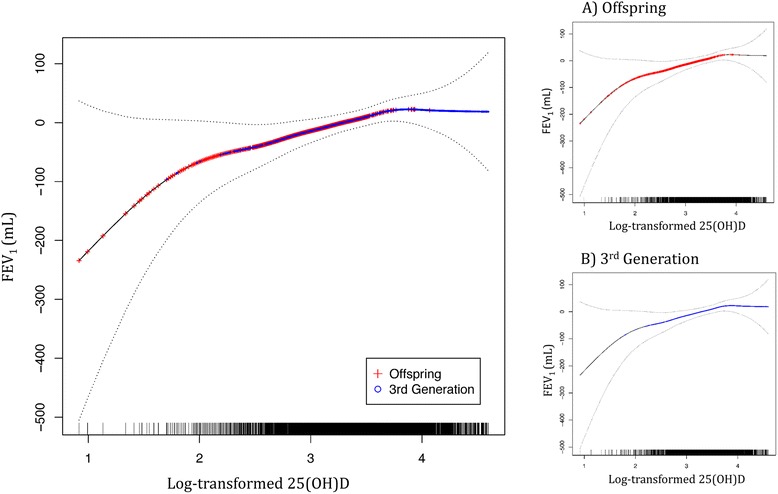
Spline analysis of log-transformed 25(OH) D by residual FEV1
We found definitive evidence of survivor bias for the longitudinal analysis: the 24 % of Offspring cohort members with both serum 25(OH)D measurements and subsequent repeated FEV1 measurements had a much lower rate of change in FEV1 compared to the 63 % of Offspring cohort members with only longitudinal lung function data (see Additional file 1 for details). Thus, we estimated the serum 25(OH)D—rate of change in FEV1 association in Third Generation cohort participants only. There was little or no association between serum 25(OH)D and rate of change in FEV1 (P = 0.97; Table 4). Neither was there any statistical evidence for a differential effect of serum 25(OH)D on rate of change in FEV1 by smoking status.
Table 4.
Association of 25(OH)D with rate of change in FEV1 (mL/yr) in the Third Generation cohort
| Third Generation Cohort | |||
|---|---|---|---|
| (N = 3,599) | |||
| Model parameterization of vitamin D: | β | SE | P |
| Continuous : Log-transformed 25(OH)D | −0.06 | 1.7 | 0.97 |
| Dichotomy 1: At risk of vitamin D deficiency (<12 ng/mL) vs. nota | −0.02 | 6.9 | 0.997 |
| Dichotomy 2: At risk of vitamin D inadequacy (<20 ng/mL) vs. not | 2.0 | 2.5 | 0.41 |
Adjusted for: baseline age, sex, height, smoking pattern over follow-up and its interaction with time, baseline pack-years, current smoking status at each time point, BMI, and month of blood draw
β beta coefficient for 25(OH)D x time effect, SE standard error, P P-value
aInterpretation: Third Generation participants at risk of vitamin D deficiency have a 0.02 mL/yr steeper rate of decline compared to Third Generation participants not at risk of deficiency
Vitamin D metabolic gene SNPs and rate of change in FEV1
We explored the association of SNPs in select vitamin D metabolic genes with rate of change in FEV1 in Offspring participants, and two genes, namely CYP27B1 and CYP2R1, were associated with rate of change in FEV1 at a nominal gene-based P < 0.05. The most significant SNP associations in CYP27B1 and CYP2R1 were for rs10877013 (P = 0.02) and rs11819875 (P = 0.004), respectively, and the minor alleles of these SNPs were associated with steeper FEV1 decline (Table 5). No other candidate genes met the threshold for gene-based statistical significance (P < 0.05), and thus none were considered further.
Table 5.
Association of the most statistically significant SNP per gene with the rate of change in FEV1 in FHS and in the meta-analyzed replication cohorts
| Gene | Gene-based P Value | Chr | Total SNPs in gene | Best SNP | Position | Coded Allelea | Freq | FHS (N = 3,230) | Meta-analyzed replication cohorts (N = 7,246) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | ||||||||
| CYP27B1 | 0.02248 | 12 | 5 | rs10877013 | 56451352 | T | 0.30 | −1.3 | 0.5 | 0.02 | −0.4 | 0.5 | 0.40 |
| CYP2R1 | 0.04417 | 11 | 15 | rs11819875 | 14873873 | G | 0.18 | −1.9 | 0.7 | 0.004 | −1.0 | 0.6 | 0.09 |
Adjusted for: baseline age, sex, height, smoking pattern over follow-up and its interaction with time, baseline smoking pack-years, and the first two principal components for genetic ancestry
Chr chromosome, SNP single nucleotide polymorphism, β beta coefficient for SNP x time effect, SE standard error, P P-value
aCoded allele and frequency for the Framingham Heart Study (FHS). All effect estimates presented in terms of FHS coded allele. Coded allele frequencies between FHS and replication cohorts were nearly identical
We explored replication of the most significant SNP in CYP27B1 and CYP2R1 with change in FEV1 in a meta-analyzed set of four independent cohorts. Rs11819875 (CYP2R1) showed a consistent direction of association with FEV1 in FHS and the meta-analyzed set of replication cohorts, but this association was not replicated by statistical significance thresholds (β = −1.0, 95 % CI: −2.2, 0.2, P = 0.09; Table 5 and Additional file 1: Table S2). Rs11819875 was associated with a lower concentration of serum 25(OH)D in data from the SUNLIGHT consortium (P = 0.15 from combined SUNLIGHT discovery and replication cohorts). Rs10877013 in CYP27B1 showed little to no association with rate of change in FEV1 in the meta-analyzed replication cohort at (β = −0.4, 95 % CI: −1.4, 0.6, P = 0.40; Table 4), and rs10877013 was associated with lower 25(OH)D in SUNLIGHT (P = 0.11 from combined SUNLIGHT discovery and replication cohorts).
Discussion
In this population-based cohort study of adults, we investigated the association of vitamin D with cross-sectional FEV1 and longitudinal FEV1, considering both serum 25(OH)D status and genetic variants in selected vitamin D metabolic genes. We demonstrated a strong, positive cross-sectional association of serum 25(OH)D with FEV1, consistent with the findings of most previously published studies [11–13, 20]. However, serum 25(OH)D was not associated with longitudinal change in FEV1 in Third Generation participants. CYP2R1, a vitamin D metabolism gene hypothesized to influence usual 25(OH)D status, was associated with longitudinal change in FEV1 in a gene-based analysis, but the most significant SNP in this gene was not replicated in a meta-analyzed set of replication cohorts.
Our cross-sectional findings provide evidence that associations between 25(OH)D and lung function may be non-linear and limited to individuals with inadequate vitamin D status. The lack of association of serum 25(OH)D with longitudinal change in FEV1 may be explained by characteristics of the study population; the Third Generation cohort was comprised of middle-aged participants with excellent serum 25(OH)D status. More than 90 % of the Third Generation cohort participants had sufficient serum 25(OH)D [25(OH)D > 20 ng/mL] and only 8.6 % and 1.2 % were considered to be at risk of inadequacy (<20 ng/mL) or deficiency (<12 ng/mL), respectively (thresholds defined by the IOM [3]). Given possible non-linear associations and/or threshold effects, our findings do not rule out a longitudinal vitamin D status—rate of change in pulmonary function association limited to persons at risk of inadequacy and/or deficiency.
Supporting this hypothesis, a recent study showed that individuals in the lowest quintile of serum 25(OH)D had a steeper rate of lung function decline compared with individuals in the highest quintile, where the mean in the highest quintile was 30 ng/mL [20]. In comparison, in our analysis of the Third Generation Framingham Heart Study cohort, the majority of the participants (>90 %) had serum 25(OH)D > 20 ng/mL. The recent finding of steeper decline limited to participants with the lowest serum 25(OH)D [20] and the lack of association in our analysis highlight the importance of considering non-linear associations and thresholds of effect defined by baseline nutritional status in future studies of 25(OH)D status and lung function.
A SNP in CYP2R1 (rs11819875) was associated with rate of change in FEV1 in FHS, but did not show statistically significant evidence for replication of the association in a meta-analysis of four independent cohort studies (P = 0.09). However, the β-coefficient for this SNP was in the same direction in 3 of the 4 cohorts, and rs11819875 was associated with lower 25(OH)D in the SUNLIGHT consortium. The P-value for replication is near the threshold, and sample size, heterogeneity in environmental factors affecting usual vitamin D status (for example, sun exposure), and/or heterogeneity in the outcome measure (rate of change in FEV1) may contribute to the lack of replication. CYP2R1 is a key hepatic 25-hydroxylase enzyme [38], and variants in this gene are consistently associated with 25(OH)D status in GWAS [5, 6]; this gene is a promising candidate for further studies investigating the role of vitamin D status in lung function phenotypes.
Our study has strengths and limitations. A limitation of the serum 25(OH)D—FEV1 analysis is that serum 25(OH)D was measured only once and a single measurement of vitamin D may not adequately estimate the long-term usual vitamin D status. We assumed that 25(OH)D measured at one point in time provided a reasonable approximation of 25(OH)D status throughout follow-up given that 25(OH)D measurements 12 months apart are highly correlated (r = 0.8 [39]) and given the relatively short follow-up period of 6–7 years. A related limitation is that supplement use data were not available, thus we could not investigate whether low serum 25(OH)D measures were associated with subsequent supplement use. While spirometry measurements in the Third Generation cohort were separated by an average of 6.1 years, a longer follow-up and more outcome measurements may be needed to identify associations between vitamin D and rate of change in FEV1. The Offspring participants included in the genetic analysis had an average spirometry follow-up of 14.7 years, leading to increased precision in the rate of decline estimates for the SNP—rate of change in FEV1 analysis. While the genetic analyses described in this study borrow concepts from the Mendelian Randomization (MR) literature [40], the limitations of the available data, including limited sample size, precluded a formal MR analysis. Overall, a major strength is the use of data from the Framingham Heart Study cohort, which provided a large, healthy, population-based sample including both men and women.
Conclusions
The findings from this study suggest that serum 25(OH)D—FEV1 associations may be limited to individuals with inadequate 25(OH)D. Further, these findings suggest important study design considerations regarding potential to benefit from nutritional advice and/or intervention in future studies of 25(OH)D and lung function. Results of the SNP—FEV1 analysis demonstrate that genetic variants in a vitamin D metabolic gene are suggestively associated with rate of change in FEV1, and our results provide evidence to guide future studies.
Acknowledgments
Funding
This research was supported by NRSA Institutional Research Training Grant T32-DK-7158-36 (JGH).
This research used data from the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. Framingham Heart Study (FHS) research was conducted in part using data and resources of the NHLBI and Boston University School of Medicine. The analyses reflect intellectual input and resource development from the FHS investigators participating in the SNP Health Association Resource (SHARe) project. This work was partially supported by NHLBI (contract no. N01-HC-25195) and its contract with Affymetrix for genotyping services (contract no. N02-HL-6-4278). A portion of this research utilized the Linux Cluster for Genetic Analysis (LinGA-II) funded by the Robert Dawson Evans Endowment of the Department of Medicine at Boston University School of Medicine and Boston Medical Center. Measurement of vitamin D in the Offspring participants was funded by NIH-NIA AG14759.
The 1994 Busselton follow-up Health Study was supported by Healthways, Western Australia. The Busselton Health Study is supported by The Great Wine Estates of the Margaret River region of Western Australia. The study gratefully acknowledges the assistance of the Western Australian DNA Bank (NHMRC Enabling Facility) with DNA samples and the support provided by The Ark at University of Western Australia for this study.
The Coronary Artery Risk Development in Young Adults (CARDIA) study was funded by contracts N01-HC-95095, N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, N01-HC-45134, N01-HC-05187, N01-HC-45205, and N01-HC-45204 from NHLBI to the CARDIA investigators. Genotyping of the CARDIA participants was supported by grants U01-HG-004729, U01-HG-004446, and U01-HG-004424 from the NHGRI. Statistical analyses were supported by grants U01-HG-004729 and R01-HL-084099 to MF.
The Cardiovascular Health Study was supported by NHLBI contracts HHSN268201200036C, N01 HHSN268200800007C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086; and NHLBI grants HL080295, HL087652, HL105756 with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided through AG023629 from the National Institute on Aging (NIA). A full list of CHS investigators and institutions can be found at https://chs-nhlbi.org/pi. The provision of genotyping data was supported in part by the National Center for Advancing Translational Sciences, CTSI grant UL1TR000124, and the National Institute of Diabetes and Digestive and Kidney Disease Diabetes Research Center (DRC) grant DK063491 to the Southern California Diabetes Endocrinology Research Center.
The Health, Aging, and Body Composition Study was supported by NIA contracts N01AG62101, N01AG2103, and N01AG62106, NIA grant R01-AG028050, NINR grant R01-NR012459, and in part by the Intramural Research Program of the NIA, NIH. The genome-wide association study was funded by NIA grant 1R01AG032098–01A1 to Wake Forest Health Sciences, and genotyping services were provided by the Center for Inherited Disease Research, which is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University, contract number HHSN268200782096C. This research was further supported by RC1AG035835.
Additional file
Table S1. Imputed SNPs in Vitamin D Metabolic Genes in FHS. Table S2. Replication cohort associations of the most significant SNPs per gene with the rate of change in FEV1.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors satisfy the requirements for authorship and contributorship. JGH and PAC designed the study and drafted the manuscript; JGH, WG, WT, JD, GTO and PAC conducted and interpreted the statistical analyses in FHS and the SNP replication study. MK, AS, SG, LRP, MF, SRH, and BMP conducted the GWAS studies used for the SNP replication lookup. SLB led a study that assayed vitamin D in FHS, which provided data on vitamin D for the analyses presented herein. The SUNLIGHT study provided lookup data on the SNP associations with vitamin D. All coauthors read and critically edited the final manuscript.
Contributor Information
JG Hansen, Email: jg553@cornell.edu.
W Gao, Email: gaowei@bu.edu.
J Dupuis, Email: dupuis@bu.edu.
GT O’Connor, Email: goconnor@bu.edu.
W Tang, Email: wt227@cornell.edu.
M Kowgier, Email: mkowgier@uhnresearch.ca.
A Sood, Email: ASood@salud.unm.edu.
SA Gharib, Email: sagharib@u.washington.edu.
LJ Palmer, Email: lyle.palmer@adelaide.edu.au.
M Fornage, Email: Myriam.Fornage@uth.tmc.edu.
SR Heckbert, Email: heckbert@u.washington.edu.
BM Psaty, Email: psaty@u.washington.edu.
SL Booth, Email: sarah.booth@tufts.edu.
Patricia A Cassano, Email: pac6@cornell.edu.
References
- 1.Minino AM. Death in the United States. NCHS Data Brief. 2011;2009:1–8. [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D, deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 3.Ross AC, Institute of Medicine (U. S.) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium.: Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 4.Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. NCHS data brief, no 5. Hyattsville: National Center for Health Statistic; 2011. Vitamin D Status: United States, 2001–2006. [PubMed] [Google Scholar]
- 5.Ahn J, Yu K, Stolzenberg-Solomon R, Simon KC, McCullough ML, Gallicchio L, et al. Genome-wide association study of circulating vitamin D levels. Hum Mol Genet. 2010;19:2739–45. doi: 10.1093/hmg/ddq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang TJ, Zhang F, Richards JB, Kestenbaum B, van Meurs JB, Berry D, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–8. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansdottir S, Monick MM, Hinde SL, Lovan N, Look DC, Hunninghake GW. Respiratory epithelial cells convert inactive vitamin D to its active form: potential effects on host defense. J Immunol. 2008;181:7090–9. doi: 10.4049/jimmunol.181.10.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bikle DD. Vitamin D, regulation of immune function. Vitam Horm. 2011;86:1–21. doi: 10.1016/B978-0-12-386960-9.00001-0. [DOI] [PubMed] [Google Scholar]
- 9.Hansdottir S, Monick MM. Vitamin D effects on lung immunity and respiratory diseases. Vitam Horm. 2011;86:217–37. doi: 10.1016/B978-0-12-386960-9.00009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfeffer PE, Hawrylowicz CM. Vitamin D and lung disease. Thorax. 2012;67:1018–20. doi: 10.1136/thoraxjnl-2012-202139. [DOI] [PubMed] [Google Scholar]
- 11.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 12.Choi CJSM, Choi WS, Kim KS, Youn SA, Lindsey T, Choi YJ, et al. Relationship Between Serum 25-Hydroxyvitamin D and Lung Function Among Korean Adults in Korea National Health and Nutrition Examination Survey (KNHANES), 2008–2010. J Clin Endocrinol Metab. 2013;98:1703–10. doi: 10.1210/jc.2012-3901. [DOI] [PubMed] [Google Scholar]
- 13.Berry DJ, Hesketh K, Power C, Hypponen E. Vitamin D status has a linear association with seasonal infections and lung function in British adults. Br J Nutr. 2011;106:1433–40. doi: 10.1017/S0007114511001991. [DOI] [PubMed] [Google Scholar]
- 14.Shaheen SO, Jameson KA, Robinson SM, Boucher BJ, Syddall HE, Aihie Sayer A, et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66:692–8. doi: 10.1136/thx.2010.155234. [DOI] [PubMed] [Google Scholar]
- 15.Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65:215–20. doi: 10.1136/thx.2009.120659. [DOI] [PubMed] [Google Scholar]
- 16.Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, et al. High doses of vitamin d to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156:105–14. doi: 10.7326/0003-4819-156-2-201201170-00004. [DOI] [PubMed] [Google Scholar]
- 17.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murdoch DR, Slow S, Chambers ST, Jennings LC, Stewart AW, Priest PC, et al. Effect of vitamin D3 supplementation on upper respiratory tract infections in healthy adults: the VIDARIS randomized controlled trial. JAMA. 2012;308:1333–9. doi: 10.1001/jama.2012.12505. [DOI] [PubMed] [Google Scholar]
- 19.Rees JR, Hendricks K, Barry EL, Peacock JL, Mott LA, Sandler RS, et al. Vitamin D3 supplementation and upper respiratory tract infections in a randomized, controlled trial. Clin Infect Dis. 2013;57:1384–92. doi: 10.1093/cid/cit549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afzal S, Lange P, Bojesen SE, Freiberg JJ, Nordestgaard BG. Plasma 25-hydroxyvitamin D, lung function and risk of chronic obstructive pulmonary disease. Thorax. 2014;69:24–31. doi: 10.1136/thoraxjnl-2013-203682. [DOI] [PubMed] [Google Scholar]
- 21.Kunisaki KM, Niewoehner DE, Singh RJ, Connett JE. Vitamin D status and longitudinal lung function decline in the Lung Health Study. Eur Respir J. 2011;37:238–43. doi: 10.1183/09031936.00146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lange NE, Sparrow D, Vokonas P, Litonjua AA. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med. 2012;186:616–21. doi: 10.1164/rccm.201110-1868OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito I, Nagai S, Hoshino Y, Muro S, Hirai T, Tsukino M, et al. Risk and severity of COPD is associated with the group-specific component of serum globulin 1 F allele. Chest. 2004;125:63–70. doi: 10.1378/chest.125.1.63. [DOI] [PubMed] [Google Scholar]
- 24.Schellenberg D, Pare PD, Weir TD, Spinelli JJ, Walker BA, Sandford AJ. Vitamin D binding protein variants and the risk of COPD. Am J Respir Crit Care Med. 1998;157:957–61. doi: 10.1164/ajrccm.157.3.9706106. [DOI] [PubMed] [Google Scholar]
- 25.Wood AM, Bassford C, Webster D, Newby P, Rajesh P. Stockley RA. Vitamin D-binding protein contributes to COPD by activation of alveolar macrophages. Thorax: Thickett DR; 2011 [DOI] [PubMed]
- 26.Horne SL, Cockcroft DW, Dosman JA. Possible protective effect against chronic obstructive airways disease by the GC2 allele. Hum Hered. 1990;40:173–6. doi: 10.1159/000153926. [DOI] [PubMed] [Google Scholar]
- 27.Chishimba L, Thickett DR, Stockley RA, Wood AM. The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax. 2010;65:456–62. doi: 10.1136/thx.2009.128793. [DOI] [PubMed] [Google Scholar]
- 28.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 29.Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–35. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 30.Artigas MS, Loth DW, Wain LV, Gharib SA, Obeidat M, Tang W, Zhai G, Zhao JH, Smith AV, Huffman JE, et al. Genome-wide association and large-scale follow up identifies 16 new loci influencing lung function. Nat Genet. 2011;43:1082–1090. doi: 10.1038/ng.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 32.Shea MK, Benjamin EJ, Dupuis J, Massaro JM, Jacques PF, D’Agostino RB, Sr, et al. Genetic and non-genetic correlates of vitamins K and D. Eur J Clin Nutr. 2009;63:458–64. doi: 10.1038/sj.ejcn.1602959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng S, Massaro JM, Fox CS, Larson MG, Keyes MJ, McCabe EL, et al. Adiposity, cardiometabolic risk, and vitamin D status: the Framingham Heart Study. Diabetes. 2010;59:242–8. doi: 10.2337/db09-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yetley EA, Pfeiffer CM, Schleicher RL, Phinney KW, Lacher DA, Christakos S, et al. NHANES monitoring of serum 25-hydroxyvitamin D: a roundtable summary. J Nutr. 2010;140:2030S–45S. doi: 10.3945/jn.110.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–9. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 36.Liu JZ, McRae AF, Nyholt DR, Medland SE, Wray NR, Brown KM, et al. A versatile gene-based test for genome-wide association studies. Am J Hum Genet. 2010;87:139–45. doi: 10.1016/j.ajhg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ligthart L, de Vries B, Smith AV, Ikram MA, Amin N, Hottenga JJ, et al. Meta-analysis of genome-wide association for migraine in six population-based European cohorts. Eur J Hum Genet. 2011;19:901–7. doi: 10.1038/ejhg.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng JB, Levine MA, Bell NH, Mangelsdorf DJ, Russell DW. Genetic evidence that the human CYP2R1 enzyme is a key vitamin D 25-hydroxylase. Proc Natl Acad Sci U S A. 2004;101:7711–5. doi: 10.1073/pnas.0402490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jorde R, Sneve M, Hutchinson M, Emaus N, Figenschau Y, Grimnes G. Tracking of serum 25-hydroxyvitamin D levels during 14 years in a population-based study and during 12 months in an intervention study. Am J Epidemiol. 2010;171:903–8. doi: 10.1093/aje/kwq005. [DOI] [PubMed] [Google Scholar]
- 40.Davey Smith G, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. 2003;32:1–22. doi: 10.1093/ije/dyg070. [DOI] [PubMed] [Google Scholar]


