Fig. 2.
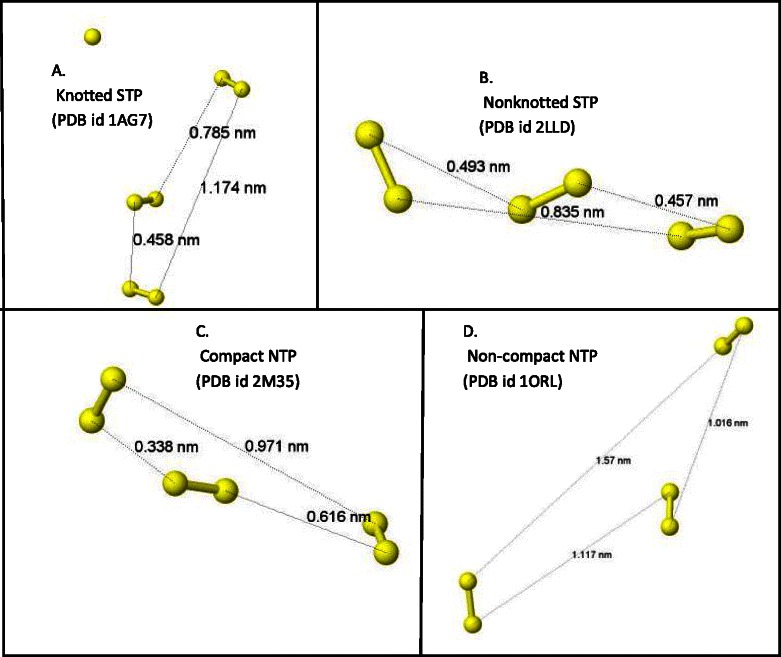
Comparison of the compactness of disulfide bonds in different types of tri-disulfide array containing peptides. Illustration of distances among the non-pairing sulfur molecules participating in the tri-disulfide array. Distances between different sulfur molecule pairs (yellow balls) were measured using jmol software. The mean of these distances indicates the average distance among the disulfide bonds demonstrating the compactness of the tri-disulfide fold in the peptide. a, b, c and d show distances of a sample representative of knotted STPs, nonknotted STPs, compact NTPs and non-compact NTPs, respectively, together with their PDB ids. The average of distance in STP toxins (a and b) is typically less than 0.85 nm, while it is more than 1.2 nm in other tri-disulfide peptides (Non-compact NTPs, data not shown) (d). Some NTPs demonstrate a similar compactness (average distance) to STPs and can be designated as compact NTPs (c)
